Home /
Expert Answers /
Chemistry /
nitrogen-and-hydrogen-gases-react-to-form-ammonia-gas-via-the-following-reaction-n-2-g-3h-2-g-pa252
(Solved): Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: N_(2)(g)+3H_(2)(g) ...
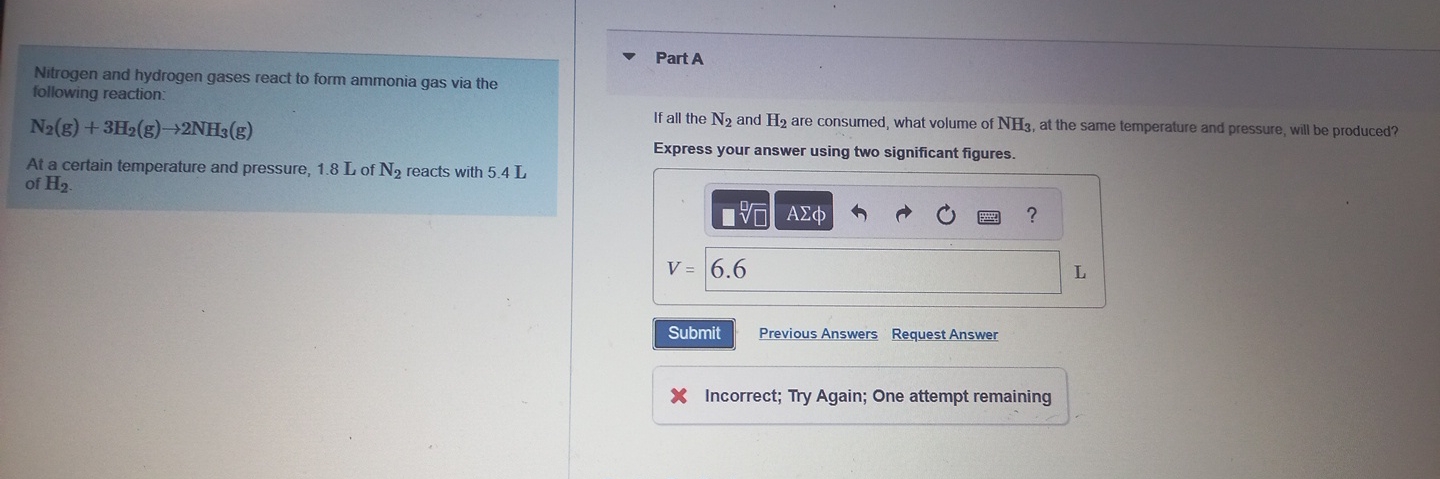
Nitrogen and hydrogen gases react to form ammonia gas via the following reaction:
N_(2)(g)+3H_(2)(g)->2NH_(3)(g)At a certain temperature and pressure,
1.8Lof
N_(2)reacts with
5.4Lof
H_(2). Part
AIf all the
N_(2)and
H_(2)are consumed, what volume of
NH_(3), at the same temperature and pressure, will be produced? Express your answer using two significant figures.
◻Request Answer Incorrect; Try Again; One attempt remaining