Home /
Expert Answers /
Chemistry /
no-matter-what-the-initial-concentrations-of-reactants-or-products-are-at-equilibrium-the-ratio-of-pa546
(Solved): No matter what the initial concentrations of reactants or products are, at equilibrium the ratio of ...
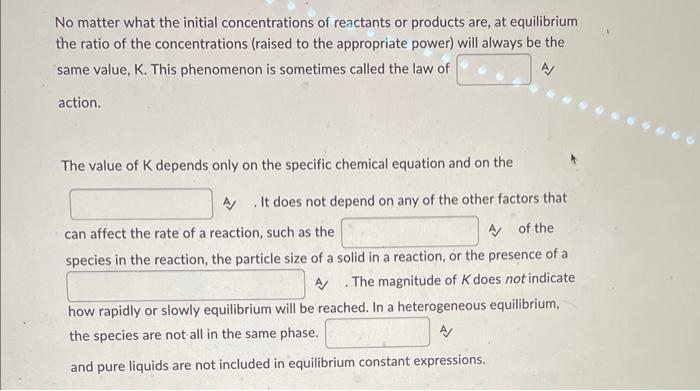
No matter what the initial concentrations of reactants or products are, at equilibrium the ratio of the concentrations (raised to the appropriate power) will always be the same value, \( \mathrm{K} \). This phenomenon is sometimes called the law of action. The value of \( \mathrm{K} \) depends only on the specific chemical equation and on the A . It does not depend on any of the other factors that can affect the rate of a reaction, such as the A) of the species in the reaction, the particle size of a solid in a reaction, or the presence of a A . The magnitude of \( K \) does not indicate how rapidly or slowly equilibrium will be reached. In a heterogeneous equilibrium, the species are not all in the same phase. and pure liquids are not included in equilibrium constant expressions.
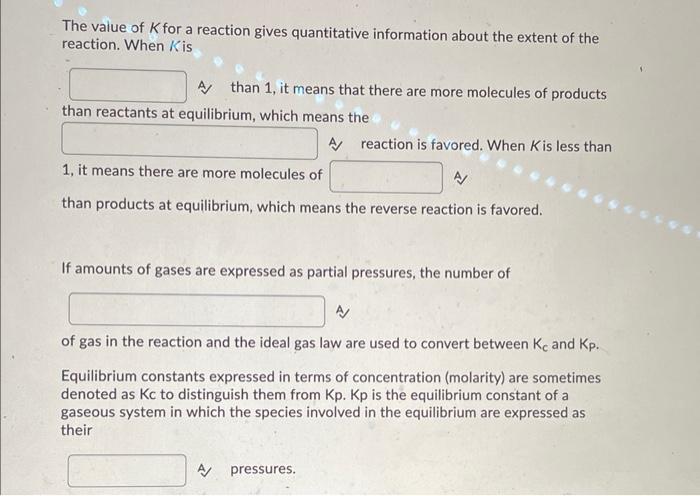
The value of \( K \) for a reaction gives quantitative information about the extent of the reaction. When \( K \) is A than 1 , it means that there are more molecules of products than reactants at equilibrium, which means the A reaction is favored. When \( K \) is less than 1 , it means there are more molecules of than products at equilibrium, which means the reverse reaction is favored. If amounts of gases are expressed as partial pressures, the number of of gas in the reaction and the ideal gas law are used to convert between \( K_{c} \) and \( K_{p} \). Equilibrium constants expressed in terms of concentration (molarity) are sometimes denoted as \( \mathrm{Kc} \) to distinguish them from \( \mathrm{Kp} \). Kp is the equilibrium constant of a gaseous system in which the species involved in the equilibrium are expressed as their A pressures.
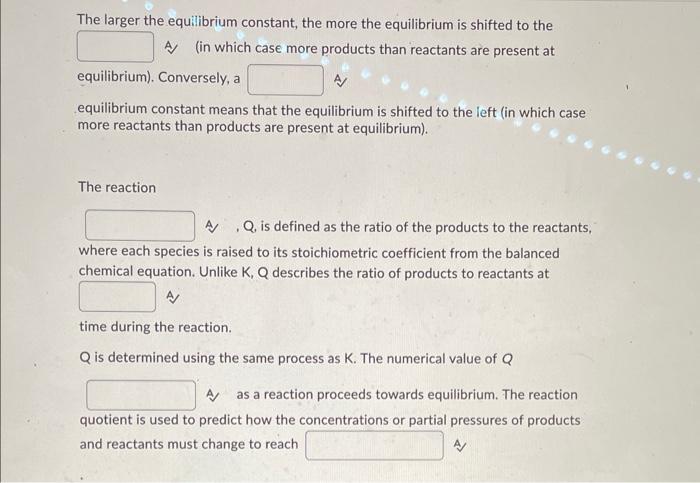
The larger the equilibrium constant, the more the equilibrium is shifted to the A) (in which case more products than reactants are present at equilibrium). Conversely, a equilibrium constant means that the equilibrium is shifted to the left (in which case more reactants than products are present at equilibrium). The reaction A , Q, is defined as the ratio of the products to the reactants, where each species is raised to its stoichiometric coefficient from the balanced chemical equation. Unlike \( K, Q \) describes the ratio of products to reactants at A/ time during the reaction. \( Q \) is determined using the same process as \( K \). The numerical value of \( Q \) A as a reaction proceeds towards equilibrium. The reaction quotient is used to predict how the concentrations or partial pressures of products and reactants must change to reach
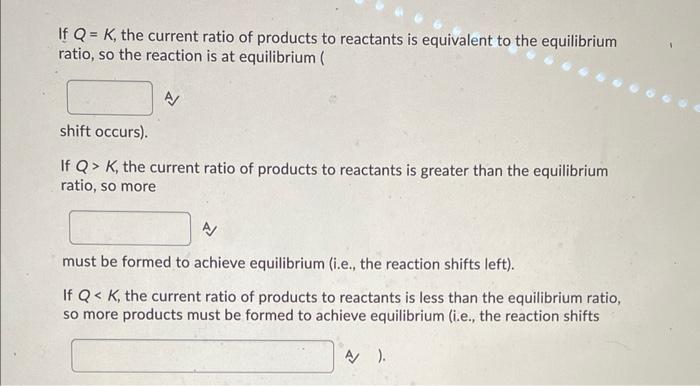
If \( Q=K \), the current ratio of products to reactants is equivalent to the equilibrium ratio, so the reaction is at equilibrium ( shift occurs). If \( Q>K \), the current ratio of products to reactants is greater than the equilibrium ratio, so more must be formed to achieve equilibrium (i.e., the reaction shifts left). If \( Q
Expert Answer
1 Mass 2 Concentration 3 Initial Concentration 4