Home /
Expert Answers /
Chemistry /
not-all-curved-arrows-used-between-resonance-structures-follow-the-patterns-seen-in-lecture-as-long-pa173
(Solved): Not all curved arrows used between resonance structures follow the patterns seen in lecture. As long ...
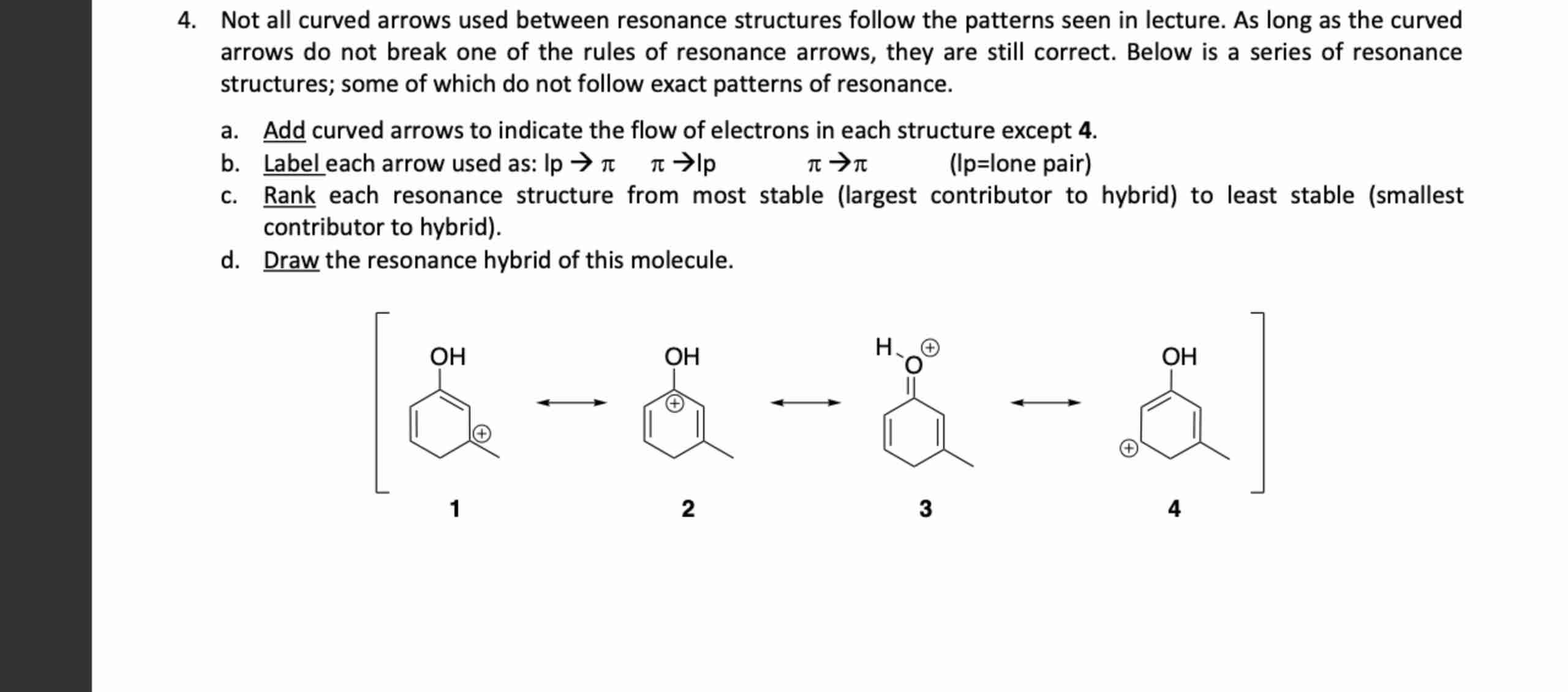
Not all curved arrows used between resonance structures follow the patterns seen in lecture. As long as the curved
arrows do not break one of the rules of resonance arrows, they are still correct. Below is a series of resonance
structures; some of which do not follow exact patterns of resonance.
a. Add curved arrows to indicate the flow of electrons in each structure except 4.
b. Label each arrow used as: lp->\pi ,\pi ->|p,\pi ->\pi , (lp=lone pair)
c. Rank each resonance structure from most stable (largest contributor to hybrid) to least stable (smallest
contributor to hybrid).
d. Draw the resonance hybrid of this molecule.