Home /
Expert Answers /
Chemistry /
o-advanced-material-1-3-na-calculating-an-equilibrium-constant-from-an-equilibrium-phosphorus-t-pa658
(Solved): O ADVANCED MATERIAL 1/3 NA Calculating an equilibrium constant from an equilibrium... Phosphorus t ...
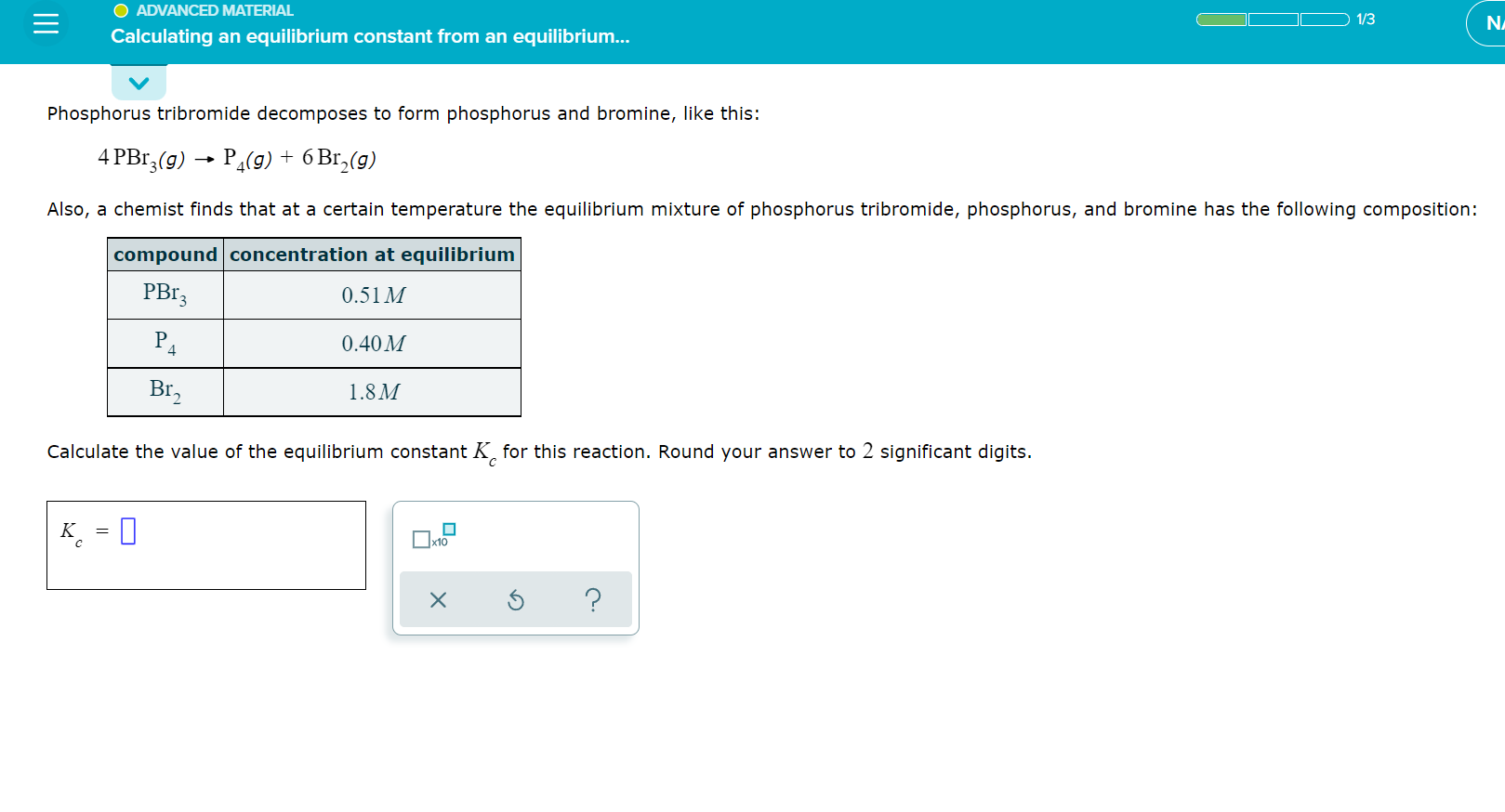
O ADVANCED MATERIAL 1/3 NA Calculating an equilibrium constant from an equilibrium... Phosphorus tribromide decomposes to form phosphorus and bromine, like this: 4 PBr3(g) ? P4(g) + 6 Br?(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of phosphorus tribromide, phosphorus, and bromine has the following composition: compound concentration at equilibrium PBr3 0.51 M P4 0.40 M Br? 1.8M Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K = ?x10 X