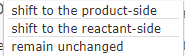Home /
Expert Answers /
Chemistry /
options-for-the-pull-down-menu-in-each-of-the-reactions-how-does-the-equilibrium-respond-to-an-in-pa395
(Solved): Options for the pull down menu In each of the reactions, how does the equilibrium respond to an in ...
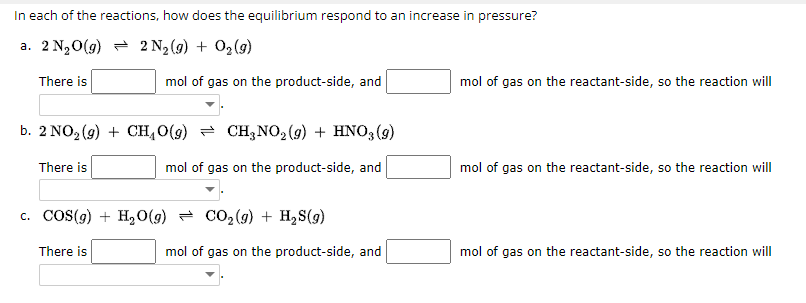
In each of the reactions, how does the equilibrium respond to an increase in pressure? a. There is mol of gas on the product-side, and of gas on the reactant-side, so the reaction will b. There is mol of gas on the product-side, and of gas on the reactant-side, so the reaction will c. There is mol of gas on the product-side, and mol of gas on the reactant-side, so the reaction will
shift to the product-side shift to the reactant-side remain unchanged