Home /
Expert Answers /
Chemistry /
oxygen-16-is-one-of-the-most-stable-nuclides-the-mass-of-a-16-mathrm-o-atom-is-15-pa810
(Solved): Oxygen-16 is one of the most stable nuclides. The mass of a \( { }^{16} \mathrm{O} \) atom is \( 15 ...
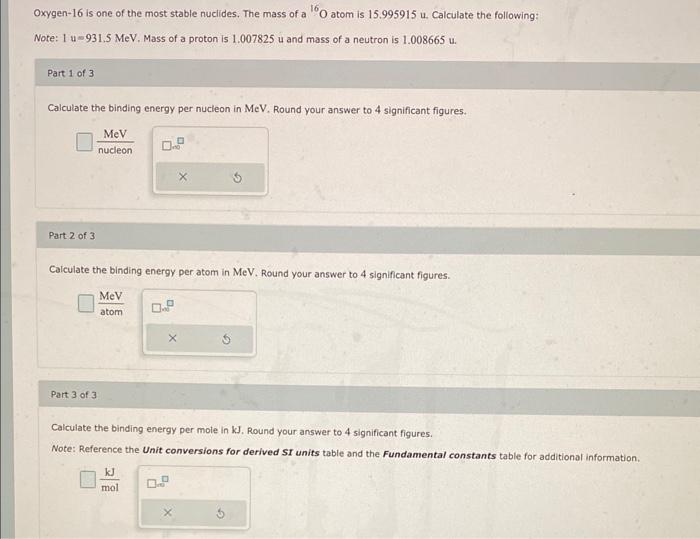
Oxygen-16 is one of the most stable nuclides. The mass of a \( { }^{16} \mathrm{O} \) atom is \( 15.995915 \mathrm{u} \). Calculate the following: Note: \( 1 \mathrm{u}=931.5 \mathrm{MeV} \), Mass of a proton is \( 1.007825 \mathrm{u} \) and mass of a neutron is \( 1.008665 \mathrm{u} \). Part 1 of 3 Calculate the binding energy per nucleon in MeV. Round your answer to 4 significant figures. Part 2 of 3 Calculate the binding energy per atom in MeV. Round your answer to 4 significant figures. Part 3 of 3 Calculate the binding energy per mole in \( \mathrm{kJ} \). Round your answer to 4 significant figures. Note: Reference the Unit conversions for derived SI units table and the Fundamental constants table for additional information. \[ \frac{\mathrm{kJ}}{\mathrm{mol}} \]