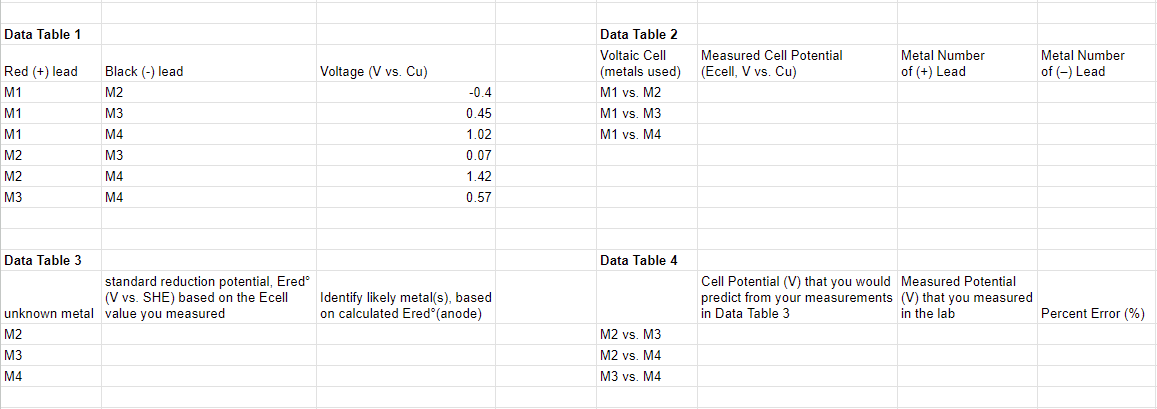
(Solved): Part A) Use the equation Ecell \deg = Ered \deg (cathode) Ered \deg and the voltages you me ...
Part A) Use the equation Ecell \deg = Ered \deg (cathode) − Ered \deg and the voltages you measured (in data table 1) above to calculate the standard reduction potential (in Volts vs. SHE) for metals M2 through M4 (showing all work). Part B) Based on the standard reduction potentials that you calculated and the standard reduction potentials listed in Appendix E, identify which metals you think M2 – M4 are. Part C) Using the identities that you’ve given to each metal and their corresponding standard reduction potential, predict the potentials (in Volts) for the remaining 3 cell combinations. Part D) Calculate the % error for each cell potential that you predicted as compared to the cell potentials that you measured. Note: M1 is copper.