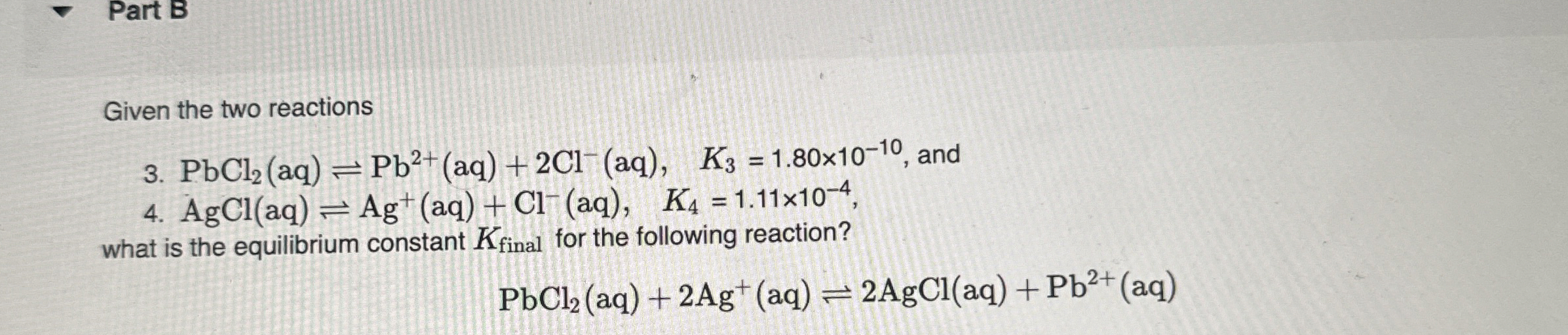Home /
Expert Answers /
Chemistry /
part-b-given-the-two-reactions-3-pbcl-2-aq-pb-2-aq-2cl-aq-k-3-1-80-times-10-10-pa905
(Solved): Part B Given the two reactions 3. PbCl_(2)(aq)Pb^(2+)(aq)+2Cl^(-)(aq),K_(3)=1.80\times 10^(-10), ...
Part B Given the two reactions 3.
PbCl_(2)(aq)⇌Pb^(2+)(aq)+2Cl^(-)(aq),K_(3)=1.80\times 10^(-10), and 4.
AgCl(aq)⇌Ag^(+)(aq)+Cl^(-)(aq),K_(4)=1.11\times 10^(-4), what is the equilibrium constant
K_(final )for the following reaction?
PbCl_(2)(aq)+2Ag^(+)(aq)⇌2AgCl(aq)+Pb^(2+)(aq)