Home /
Expert Answers /
Chemistry /
parta-submit-request-answer-part-b-submat-reguestanswer-part-c-submit-reguestnswer-name-each-of-pa132
Expert Answer
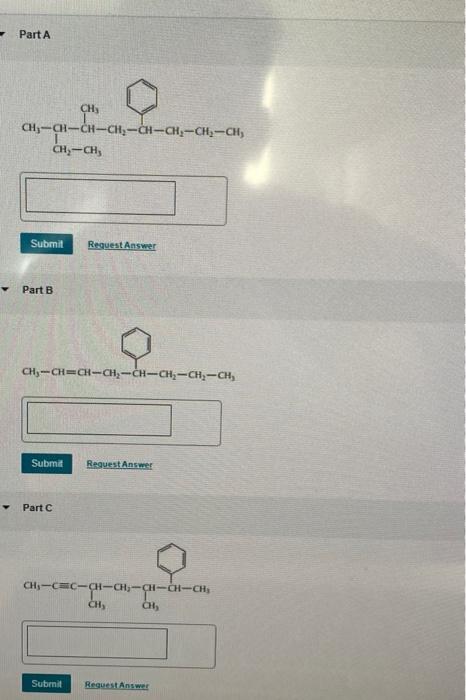
The IUPAC rules of naming alkanes:???Identify the longest continuous carbon chain in the molecule. This will serve as the parent chain.???Number the carbon atoms in the parent chain to give the lowest possible set of locants (numbers) to substituents.???Identify and name any substituent groups attached to the parent chain. Common substituents include methyl (CH3-), ethyl (C2H5-), propyl (C3H7-), etc.???Indicate the position of each substituent group on the parent chain using the locants assigned in step 2. Use commas to separate multiple locants, and use hyphens to separate locants from the substituent names.???Arrange the substituent names alphabetically. If there are multiple substituents of the same kind, use numerical prefixes (di-, tri-, tetra-, etc.) to indicate their number.???If there are multiple identical substituents, use prefixes such as "di-", "tri-", etc., to indicate their number. These prefixes do not affect the alphabetical order of the substituent names.???If there are multiple substituent groups of different kinds, arrange them in alphabetical order and assign them the lowest possible locants.???If there are multiple parent chains of equal length, choose the one that has more substituents. If the substituents are the same, choose the parent chain with substituents at the lowest-numbered carbon positions.Part A : The name of the given compound is 1. The main parent carbon chain consists of nine carbon atoms 2. It contains three substituents i.e., two methyl groups and a phenyl group at positions 6, 7, and 3 respectively.