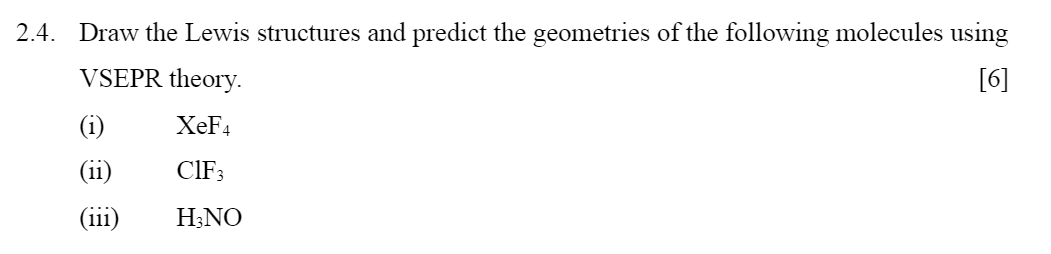Home /
Expert Answers /
Chemistry /
please-answer-all-questions-1-3-2-draw-the-lewis-structures-and-predict-the-geometries-of-the-follo-pa259
Expert Answer
In a represent the atoms, and dots or lines are used to represent valence electrons. The dots are placed around the symbol to represent the electrons in the outermost energy level (valence electrons) of the atom. these dots are arranged in pairs, with each pair representing a lone pair of electrons. Lines are used to represent covalent bonds, which aroutermost energy level (valence electrons) e formed when two