Home /
Expert Answers /
Chemistry /
please-help-consider-these-reactions-where-m-represents-a-generic-metal-1-2m-s-6hcl-aq-2mcl3-pa224
(Solved): Please help Consider these reactions, where M represents a generic metal. 1. 2M(s)+6HCl(aq)2MCl3 ...
Please help
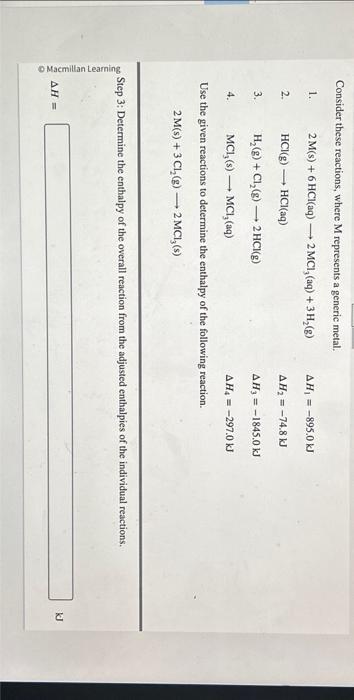

Consider these reactions, where represents a generic metal. 1. 2. 3. 4. Use the given reactions to determine the enthalpy of the following reaction. Step 3: Determine the enthalpy of the overall reaction from the adjusted enthalpies of the individual reactions.
Expert Answer
Answer:-Needed ultimate reaction equation is- Given reactions, ----...