Home /
Expert Answers /
Chemistry /
please-help-time-sensitive-the-following-initial-rate-data-are-for-the-reaction-of-mercury-ii-ch-pa824
(Solved): please help! time sensitive!! The following initial rate data are for the reaction of mercury(II) ch ...
please help! time sensitive!!
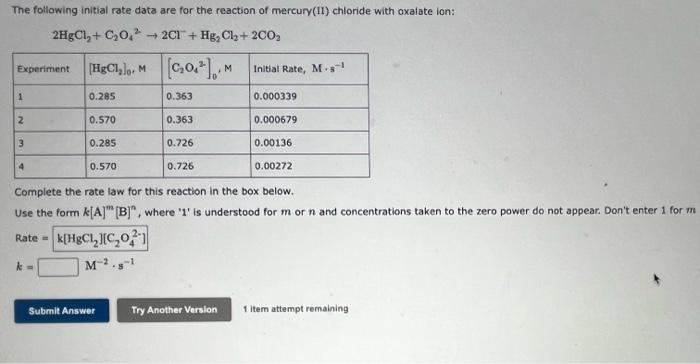

The following initial rate data are for the reaction of mercury(II) chloride with oxalate ion: \[ 2 \mathrm{HgCl}_{2}+\mathrm{C}_{2} \mathrm{O}_{4}{ }^{2} \rightarrow 2 \mathrm{Cl}^{-}+\mathrm{Hg}_{2} \mathrm{Cl}_{2}+2 \mathrm{CO}_{2} \] Complete the rate law for this reaction in the box below. Use the form \( k[\mathbf{A}]^{m}[\mathbf{B}]^{n} \), where '1' is understood for \( m \) or \( n \) and concentrations taken to the zero power do not appear. Don't enter 1 for \( m \) Rate \( = \) \[ k=\quad \mathrm{M}^{-2} \cdot \mathrm{s}^{-1} \]