Home /
Expert Answers /
Advanced Physics /
please-nswer-with-steps-question-2-consider-a-particle-of-mass-do-not-use-m-otherwise-will-confuse-pa569
(Solved): please nswer with steps Question 2 Consider a particle of mass (do not use m, otherwise will confuse ...
please nswer with steps
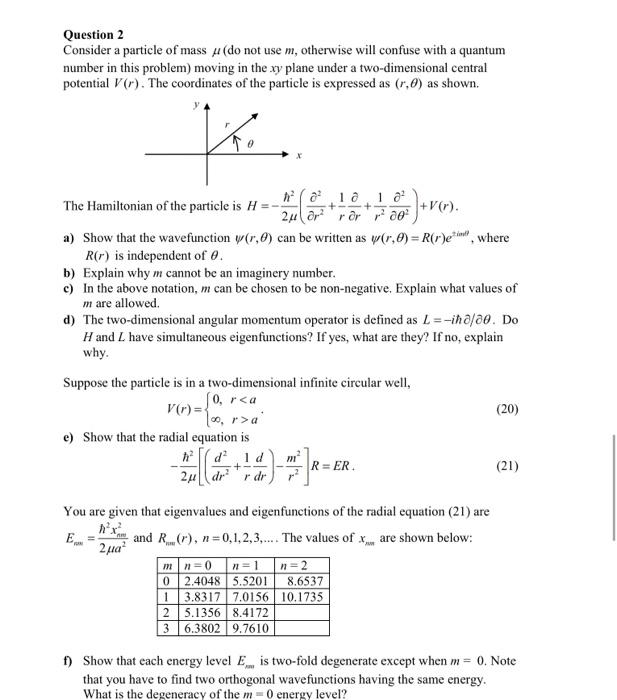



Question 2 Consider a particle of mass (do not use m, otherwise will confuse with a quantum number in this problem) moving in the xy plane under a two-dimensional central potential V(r). The coordinates of the particle is expressed as (r.0) as shown. K The Hamiltonian of the particle is H =-; + 18 18² ²00² + +v(). 2? r² ror a) Show that the wavefunction (r,) can be written as (,0) = R(r)e, where R(r) is independent of 0. b) Explain why m cannot be an imaginery number. c) In the above notation, m can be chosen to be non-negative. Explain what values of m are allowed. d) The two-dimensional angular momentum operator is defined as L=-iho/00. Do H and I have simultaneous eigenfunctions? If yes, what are they? If no, explain why. Suppose the particle is in a two-dimensional infinite circular well, V(r)= [0, ra' (20) e) Show that the radial equation is d² 1 d 2-[(4+¹4)-7]R= R=ER. (21) 2? dr² r dr You are given that eigenvalues and eigenfunctions of the radial equation (21) are E = and R (r), n=0,1,2,3,.... The values of x, are shown below: 2 ??? mn=0 n=1 n=2 0 2.4048 5.5201 8.6537 1 3.8317 7.0156 10.1735 2 5.1356 8.4172 3 6.3802 9.7610 f) Show that each energy level E is two-fold degenerate except when m = 0. Note that you have to find two orthogonal wavefunctions having the same energy. What is the degeneracy of the m=0 energy level?
g) We use the notation s, p, d, f,... to denote states with angular momentum quantum number m = 0, 1, 2, 3, ... respectively. What are the five lowest energy states in this notation (for example, a state with n=0 and m=0 should be denoted as Os )? List them in increasing order of energy. continue on next page... h) Suppose we put two identical non-interacting spinless (S = 0) particles in this circular well, what are the energies and normalized total wavefunctions Y(?,0,2,0?) in the ground state (lowest energy) and first excited state (next lowest energy) respectively. You should express the total wavefunctions in terms of R i) Repeat part h) if the two particles are electrons, again assuming that they are non- interacting, i.e., ignore electron-electron repulsion. Remember that since the spin is nonzero, wavefunctions have both orbit and spin parts. j) Discuss how electron-electron repulsion splits the first excited state in part i). Give a qualitative reason why one has higher energy than the other. No mathematical expression or proof is necessary. Now consider a particle in a three-dimensional infinite cylindrical well, i.e., it is inside a cylinder which is infinitely long along the z direction and has radius a in the x-y plane. k) Find its energies and wavefunctions (r.0.z) using your knowledge of the two- dimensional infinite circular well above. Give conditions and allowed values for any variable or quantum number you introduce. Are the energy levels discrete or continuous? Are the wavefunctions normalizable?