Home /
Expert Answers /
Chemistry /
please-show-all-steps-thank-you-nbsp-1-if-an-empty-50-0l-container-is-filled-with-1-00mol-of-coc-pa729
(Solved): Please show all steps, thank you. 1) if an empty 50.0L container is filled with 1.00mol of COC ...
Please show all steps, thank you.
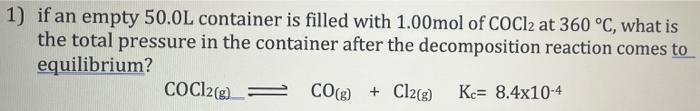
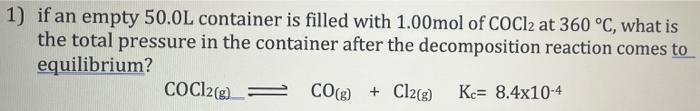
1) if an empty 50.0L container is filled with 1.00mol of COCl2 at 360 °C, what is the total pressure in the container after the decomposition reaction comes to equilibrium? COC12(g) = CO(g) + Cl2(g) Kc= 8.4x10-4