Home /
Expert Answers /
Chemistry /
please-show-all-work-2-suppose-thal-1-15g-of-rubbing-alcohol-c3h8o-evaporates-from-a-65-0-pa933
(Solved): please show all work 2. Suppose thal 1.15g of rubbing alcohol (C3H8O) evaporates from a 65.0 ...
please show all work
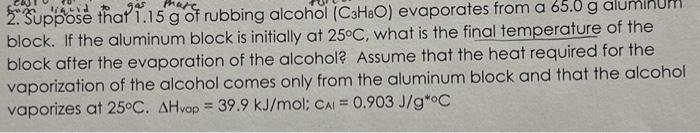
2. Suppose thal of rubbing alcohol evaporates from a aluminum block. If the aluminum block is initially at , what is the final temperature of the block after the evaporation of the alcohol? Assume that the heat required for the vaporization of the alcohol comes only from the aluminum block and that the alcohol vaporizes at
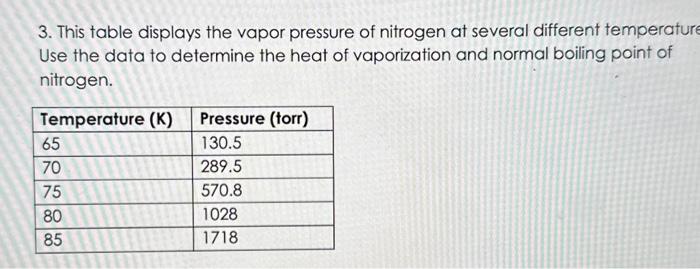
3. This table displays the vapor pressure of nitrogen at several different temperatur Use the data to determine the heat of vaporization and normal boiling point of nitrogen.