Home /
Expert Answers /
Chemistry /
pleases-help-procedures-water-preparation-c-repeat-the-procedure-using-50-0-mathrm-ml-be-pa953
(Solved): pleases help Procedures Water Preparation: C: Repeat the procedure, using \( 50.0 \mathrm{~mL} \) Be ...
pleases help


Procedures Water Preparation: C: Repeat the procedure, using \( 50.0 \mathrm{~mL} \) Because carbon dioxide from the air \( \quad \) from step B, diluting to \( 50.0 \mathrm{~mL} \). This dissolves in water, yielding an acidic time pour \( 10.0 \mathrm{~mL} \) samples into test solution, we must remove all dissolved \( \quad \) tubes \( \# 3 \) and \( \# 4 \). carbon dioxide from the water used in this experiment. Place 350 to \( 400 \mathrm{~mL} \) of \( \quad \) D: Dilute \( 5.0 \mathrm{~mL} \) of solution from step C deionized water into a beaker and heat to \( 50.0 \mathrm{ml} \) in the same fashion and pour it to boiling using a hot plate. Set the \( \quad 10.0 \mathrm{~mL} \) into test tube \( \# 5 \). hot plate to \( 300-400 \) to start the water boiling and then turn down the E: Finally, dilute \( 5.0 \mathrm{~mL} \) from step \( D \) to temperature. Continue boiling for \( \quad 50.0 \mathrm{~mL} \) and pour \( 10.0 \mathrm{~mL} \) into test tube approximately 10 minutes and then \( \quad \# 6 \). remove the beaker from the hot plate, cover with a large watch glass and allow Assuming complete disassociation of to cool to room temperature. \( \quad \) the acid, calculate the hydronium Preparation of Standard Solutions: \( \quad \) and the \( \mathrm{pH} \). Then record your data. While the deionized water is being prepared, wash and label six test tubes. To each of the test tube numbers 1-3, Label them 1 to 6 . When the deionized add 2 drops of thymol blue indicator and water is room temperature, prepare a \( \quad \) mix well using a vortexer. Note the set of solutions from the following steps. color of each of the tubes and record your observations. From these A: As accurately as possible, measure observations, estimate the \( \mathrm{pH} \) range of \( 5.0 \mathrm{~mL} \) of \( 1.0 \mathrm{M} \mathrm{HCl} \) into a \( 50.0 \mathrm{~mL} \quad \) thymol blue. graduated cylinder. Your \( 5.0 \mathrm{~mL} \) measurement will be more accurate if \( \quad \) To test tubes 4-6, add 2 drops of methyl you do it in a separate \( 10.0 \mathrm{~mL} \quad \) orange indicator and mix well using a graduated cylinder then transfer it to the vortexer. Note the color of each of the \( 50.0 \mathrm{~mL} \) graduated cylinder. Then dilute test tube and record your observations. the \( \mathrm{HCl} \) to \( 50.0 \mathrm{~mL} \) using the treated \( \quad \) From these observations, estimate the deionized water. Transfer this solution \( \quad \mathrm{pH} \) range of methyl orange. into a small dry beaker and stir thoroughly with a glass rod. Pour \( 10.0 \mathrm{~mL} \) of this solution into test tube \#1. B: Carefully and accurately pour \( 5.0 \mathrm{~mL} \) of the solution made in step A into a deionized rinsed \( 50.0 \mathrm{~mL} \) graduated cylinder and again dilute to the \( 50.0 \mathrm{~mL} \) with the treated deionized water. Transfer to a clean dry beaker, stir, and pour \( 10.0 \mathrm{~mL} \) into test tube \#2.
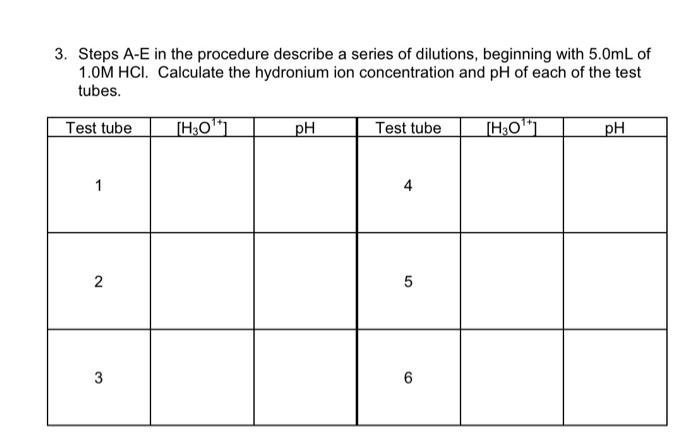
3. Steps A-E in the procedure describe a series of dilutions, beginning with \( 5.0 \mathrm{~mL} \) of \( 1.0 \mathrm{M} \mathrm{HCl} \). Calculate the hydronium ion concentration and \( \mathrm{pH} \) of each of the test tubes.
Expert Answer
The formula for hydronium ion concentration is as follows: For test tube 1: 5.0 mL of 1.0 M HCl solution is diluted to 50.0 mL. Calculate the hydronium ion concentration after dilution, as follows: Calculate the pH, as follows: For