Home /
Expert Answers /
Chemistry /
pls-answer-for-the-reaction-2-mathrm-h-2-mathrm-s-mathrm-g-rightarrow-2-mathrm-h-2-pa757
(Solved): pls answer For the reaction \( 2 \mathrm{H}_{2} \mathrm{~S}(\mathrm{~g}) \rightarrow 2 \mathrm{H}_{2 ...
pls answer



For the reaction \( 2 \mathrm{H}_{2} \mathrm{~S}(\mathrm{~g}) \rightarrow 2 \mathrm{H}_{2}(\mathrm{~g})+\mathrm{S}_{2}(\mathrm{~g}), \mathrm{K}_{\mathrm{p}}=1.5 \times 10^{-5} \) at \( 800.0^{\circ} \mathrm{C} \). If the initial partial pressure of \( \mathrm{H}_{2} \) and \( \mathrm{S}_{2} \) in a closed container are \( 4.00 \mathrm{~atm} \) and \( 2.00 \mathrm{~atm} \), respectively, what is the approximate equilibrium partial pressure of \( \mathrm{H}_{2} \mathrm{~S} \) ?(round to two decimals, after writing the number, leave a space and write the units)
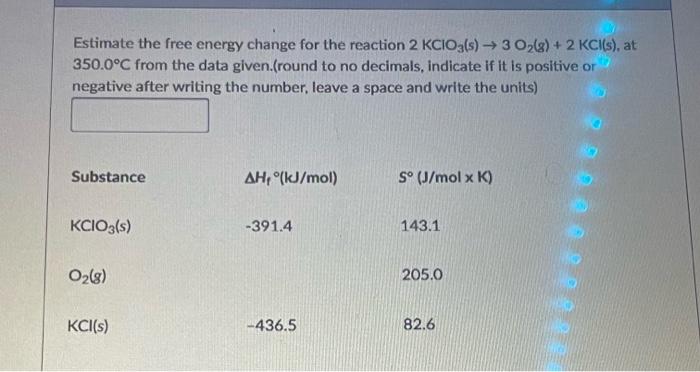
Estimate the free energy change for the reaction \( 2 \mathrm{KClO}_{3}(\mathrm{~s}) \rightarrow 3 \mathrm{O}_{2}(\mathrm{~g})+2 \mathrm{KCl}(\mathrm{s}) \), at \( 350.0^{\circ} \mathrm{C} \) from the data given.(round to no decimals, indicate if it is positive or negative after writing the number, leave a space and write the units)
The change in standard molar entropy, \( \Delta 5^{\circ} \) for the reaction \( 2 \mathrm{KClO}_{3}(s) \rightarrow 2 \mathrm{KCl}(\mathrm{s})+3 \) \( \mathrm{O}_{2}(\mathrm{~g}) \) is \( 494.0 \mathrm{~J} / \mathrm{mol} \times \mathrm{K} \) at \( 25.00^{\circ} \mathrm{C} \). What is the standard molar entropy of \( \mathrm{O}_{2}(\mathrm{~g}) \) at \( 25.00^{\circ} \mathrm{C} \) ?(round to no decimals, Indicate if it is positive or negative after writing the number, leave a space and write the unitsi) \[ \begin{array}{ll} S^{\circ}\left(\mathrm{KClO}_{3}, \mathrm{~s}\right) & =143.1 \mathrm{~J} / \mathrm{mol} \cdot \mathrm{K} \\ S^{\circ}\left(\mathrm{KCl}^{\mathrm{s}}\right) & =82.6 \mathrm{~J} / \mathrm{mol} \cdot \mathrm{K} \end{array} \]