Home /
Expert Answers /
Chemistry /
pls-help-nbsp-determine-the-cell-potential-ecell-of-an-electrochemical-cell-based-on-the-followin-pa789
(Solved): pls help Determine the cell potential (Ecell) of an electrochemical cell based on the followin ...
pls help
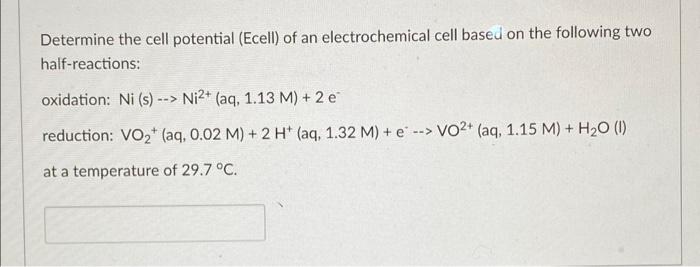

Determine the cell potential (Ecell) of an electrochemical cell based on the following two half-reactions: oxidation: Ni (s) --> Ni2+ (aq, 1.13 M) + 2 e reduction: Vozt (aq, 0.02 M) + 2 H+ (aq, 1.32 M) + e --> VO2+ (aq, 1.15 M) + H2O (1) > at a temperature of 29.7 °C.
Expert Answer
Ni(s) --------------> Ni^2+(aq) + 2e^- E0 = 0.23v 2VO2^+(aq) + 4H^+(aq) + 2e^- ----------> 2VO^2+(aq) + 2H2O(l) E0 =