(Solved): Practice Problems How many significant figures are in each of the measurements? Na a. 125 ft d. 4\ti ...
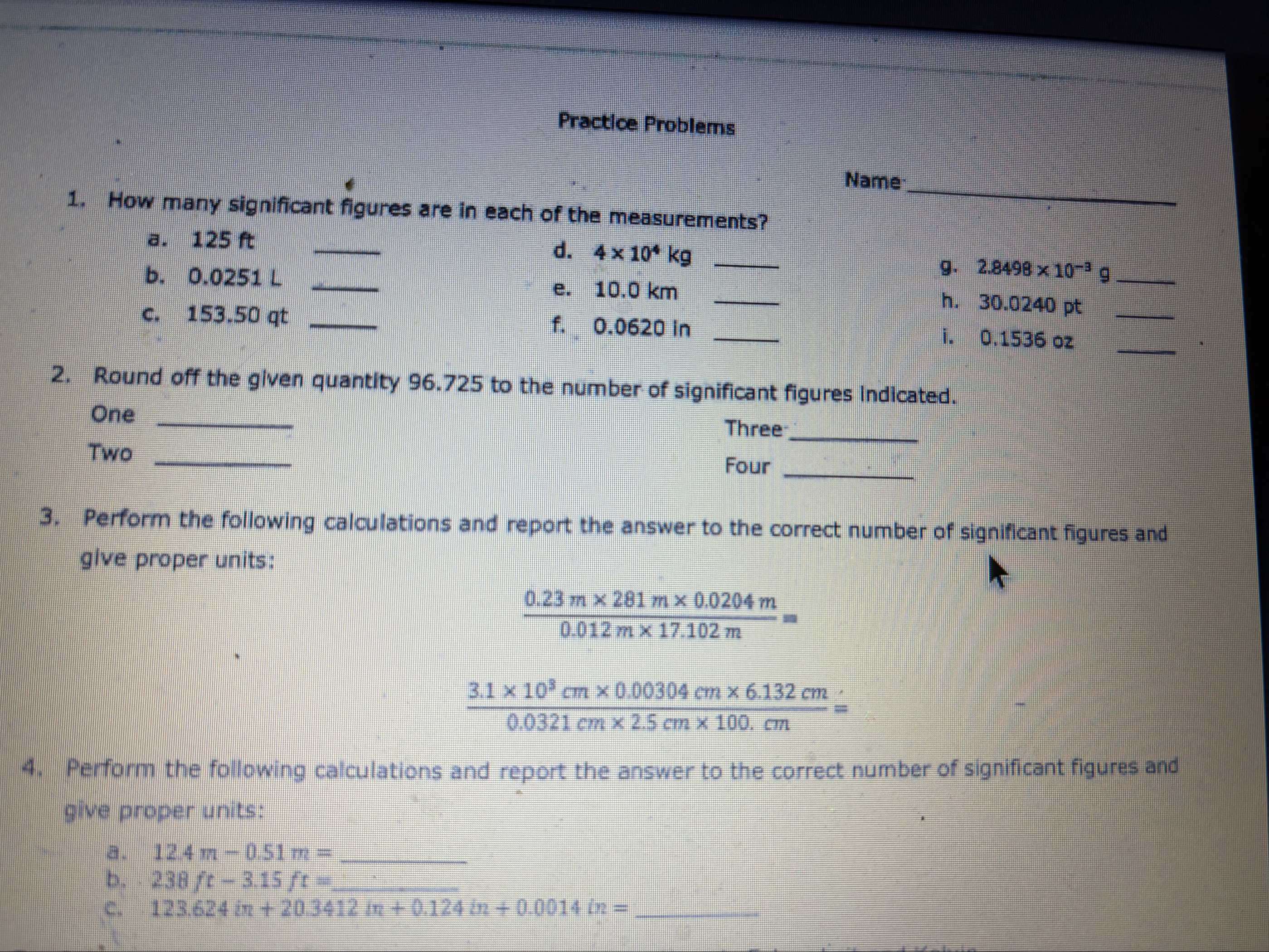
Practice Problems How many significant figures are in each of the measurements? Na a. 125 ft d.
4\times 10^(4)kgb. 0.0251 L
q,g.
2.8498\times 10^(-3)gc. 153.50 qt
◻e. 10.0 km f. 0.0620 in h. 30.0240 pt i. 0.1536 oz Round off the given quantity 96.725 to the number of significant figures indicated. One Two
◻Three Four 3. Perform the following calculations and report the answer to the correct number of significant figures and give proper units:
(0.23(m)\times 281(m)\times 0.0204(m))/(0.012(m)\times 17.102(m))=
(3.1\times 10^(3)(cm)\times 0.00304(cm)\times 6.132(cm))/(0.0321(cm)\times 2.5(cm)\times 100(cm))=Perform the following calculations and report the answer to the correct number of significant figures and give proper units: a.
12.4m-0.51m=
q,b.
238ft-3.15ft=
q,c.
123.624in 20.3412in 0.124in 0.0014in=
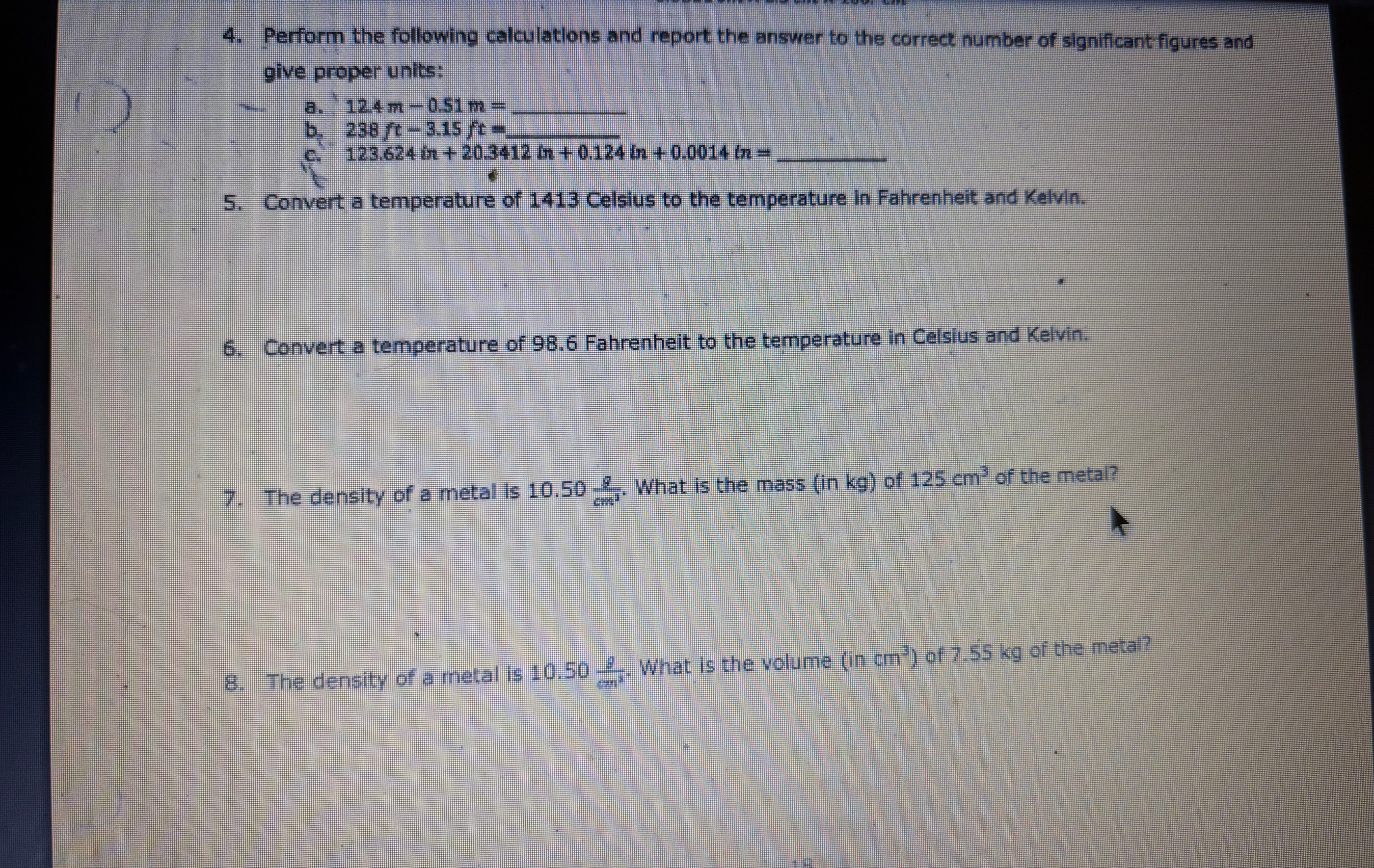
q,Perform the following calculations and report the answer to the correct number of significant figures and give proper units: a.
12.4m-0.51m=
q,b.
238ft-3.15ft=
q,c.
123.624m 20.3412m 0.124m 0.0014m=
q,Convert a temperature of 1413 Celsius to the temperature in Fahrenheit and Kelvin. Convert a temperature of 98.6 Fahrenheit to the temperature in Celsius and Kelvin. The density of a metal is
10.50((g))/(cm^(3)). What is the mass (in kg ) of
125cm^(3)of the metal? The density of a metal is
10.50((g))/(m^(3)). What is the volume
(incm^(3))of 7.55 kg of the metal?e) 925 g
q,mg
◻ng
q,b f) 3245 ft
◻cm
◻km
◻mile g) 65.2 L
◻
cm^(3)
◻
◻gal h)
5.65ft^(3)
◻
yd^(3)
◻
cm^(3)
◻
mm^(3)i) 215 mL
◻
m^(3)
◻
mm^(3)
◻
ft^(3)j) 645 L
◻
cm^(3)
q,gal
q,pt k) 239 mL
◻
cm^(3)
◻L
◻1.25 L
q,
cm^(9)
◻
dm^(3)
◻t m) 0.0645 kg
q,g
◻Mg
◻Ib Convert 125 ounces per cubic foot (oz/t
^(3)) to centigrams per cubic millimeter (og/mm
^(3)).