Home /
Expert Answers /
Chemistry /
pre-equilibria-conditions-in-reaction-mechanisms-co-g-cl-2-g-gt-cocl-2-g-the-above-reaction-pa234
(Solved): Pre-Equilibria Conditions in Reaction Mechanisms CO(g)+Cl_(2)(g)->COCl_(2)(g) The above reaction ...
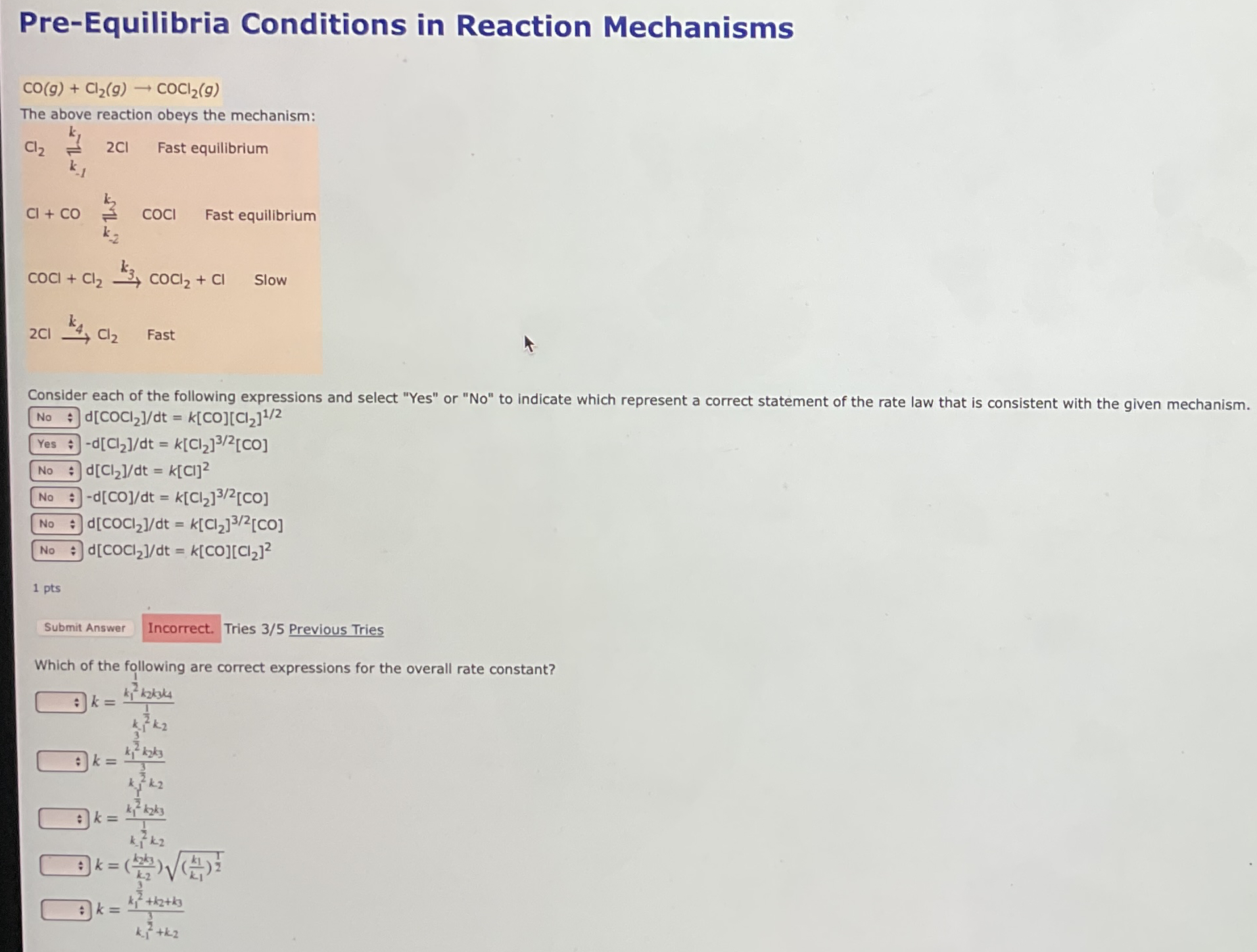
Pre-Equilibria Conditions in Reaction Mechanisms
CO(g)+Cl_(2)(g)->COCl_(2)(g)The above reaction obeys the mechanism:
Cl_(2)⇌_(_(-1))^(k_(1))2Cl,Fast equilibrium
+CO⇌_(k_(-2))^(k_(2))COCl,Fast equilibrium
COCl+Cl_(2)->k_(3)COCl_(2)+Cl, Slow
2Cl->k_(4)Cl_(2),Fast Consider each of the following expressions and select "Yes" or "No" to indicate which represent a correct statement of the rate law that is consistent with the given mechanism.
◻
d(COCl_(2))/(d)t=k[CO][Cl_(2)]^((1)/(2))
◻
-d(Cl_(2))/(d)t=k[Cl_(2)]^((3)/(2))[CO]
◻
d(Cl_(2))/(d)t=k[Cl]^(2)
◻
-d(CO)/(d)t=k[Cl_(2)]^((3)/(2))[CO]
◻
d(COCl_(2))/(d)t=k[Cl_(2)]^((3)/(2))[CO]
◻
|d(COCl_(2))/(d)t||
=k[CO][Cl_(2)]^(2)1 pts
◻
◻Tries 3/5 Previous Tries Which of the following are correct expressions for the overall rate constant? k
k=(k_(1)^((1)/(2))k_(2)k_(3)4_(4))/(k_(k_(1))^((1)/(2))k_(2))
k
k=(k_(1)^((3)/(2))k_(2k)k_(3))/(k_(1)^((3)/(2))k_(2))
k
k=(k_(1)^((1)/(2))k_(2)k_(3))/(k_(-1)^(k_(2)^(2))k_(2))
◻
k=((k_(2)k_(3))/(k_(2)))\sqrt(((k_(1))/(k_(1)))^((1)/(2)))
◻
k=(k_(1)^((3)/(2))+k_(2)+k_(3))/(k_(-1)^((3)/(2))+k_(2))