Home /
Expert Answers /
Electrical Engineering /
problem-5-the-energy-diagram-of-a-4-level-laser-system-is-presented-on-the-figure-below-in-this-sy-pa640
(Solved): Problem 5: The energy diagram of a 4-level laser system is presented on the figure below. In this sy ...
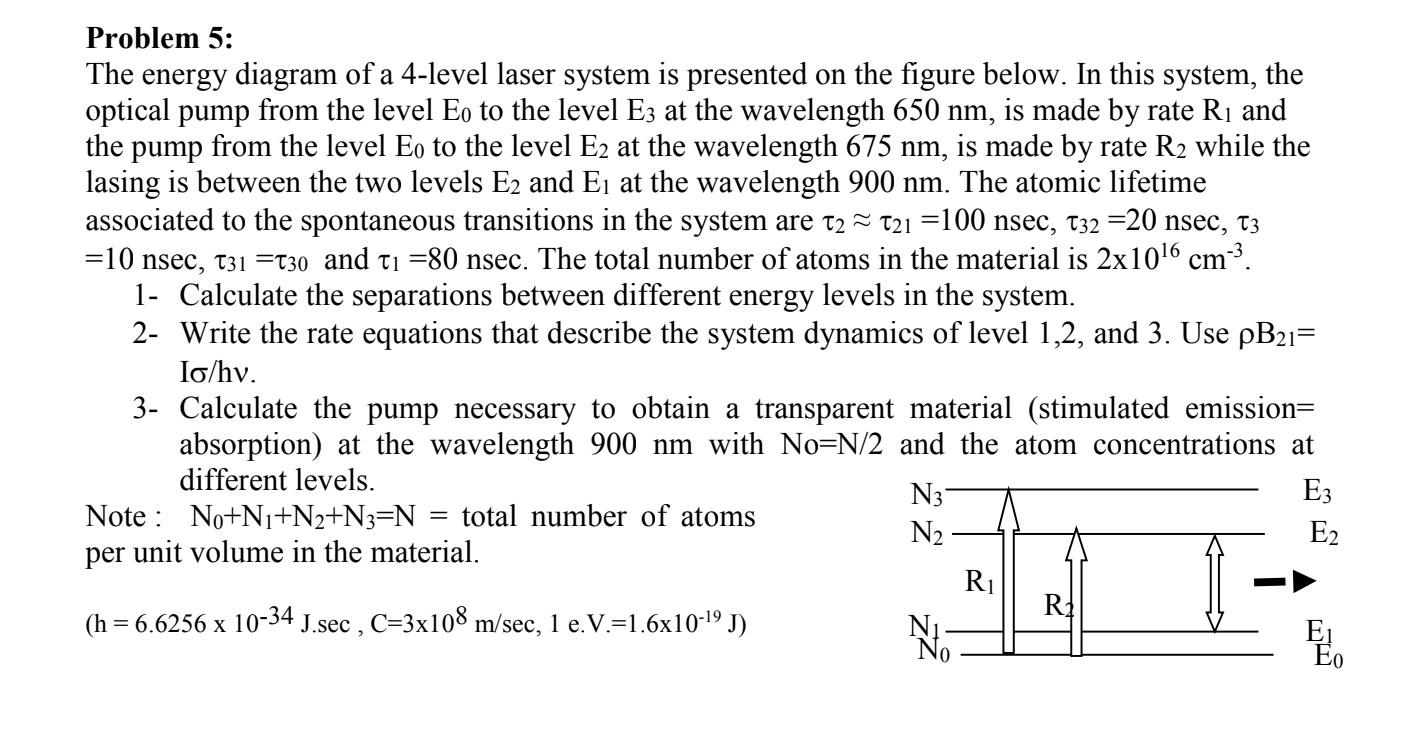
Problem 5:
The energy diagram of a 4-level laser system is presented on the figure below. In this system, the
optical pump from the level E_(0) to the level E_(3) at the wavelength 650 nm , is made by rate R_(1) and
the pump from the level E_(0) to the level E_(2) at the wavelength 675 nm , is made by rate R_(2) while the
lasing is between the two levels E_(2) and E_(1) at the wavelength 900 nm . The atomic lifetime
associated to the spontaneous transitions in the system are \tau _(2)~~\tau _(21)=100nsec,\tau _(32)=20nsec,\tau _(3)
=10nsec,\tau _(31)=\tau _(30) and \tau _(1)=80nsec. The total number of atoms in the material is 2\times 10^(16)cm^(-3).
1- Calculate the separations between different energy levels in the system.
2- Write the rate equations that describe the system dynamics of level 1,2 , and 3 . Use \rho B_(21)=
I(\sigma )/(h)v.
3- Calculate the pump necessary to obtain a transparent material (stimulated emission=
absorption) at the wavelength 900 nm with No=(N)/(2) and the atom concentrations at
different levels.
Note : N_(0)+N_(1)+N_(2)+N_(3)=N= total number of atoms
per unit volume in the material.
(h=6.6256\times 10^(-34)(J).sec,C=3\times 10^(8)(m)/(sec,1 e.V. )=1.6\times 10^(-19)(J))