Home /
Expert Answers /
Mechanical Engineering /
problem-hw4-3-a-gas-initially-at-2-8-bar-and-60-deg-c-is-compressed-to-a-final-pressure-of-14-bar-in-pa950
(Solved): Problem HW4.3 A gas initially at 2.8 bar and 60\deg C is compressed to a final pressure of 14 bar in ...
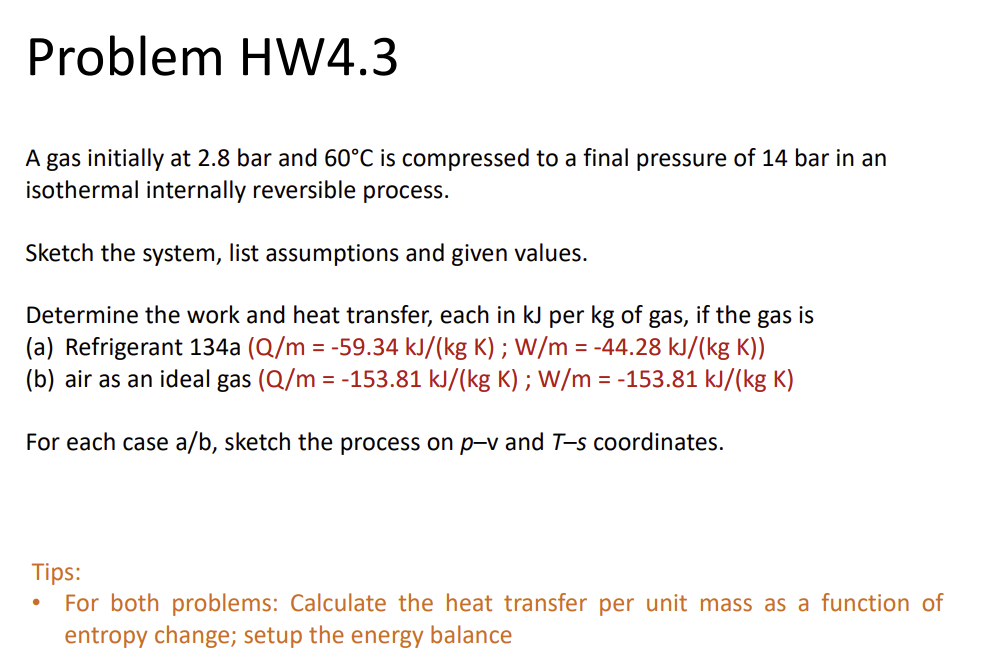
Problem HW4.3
A gas initially at 2.8 bar and 60\deg C is compressed to a final pressure of 14 bar in an isothermal internally reversible process.
Sketch the system, list assumptions and given values.
Determine the work and heat transfer, each in kJ per kg of gas, if gas is
(a) Refrigerant 134a ((Q)/(m)=-59.34 (kJ)/(kg*K); (W)/(m)=-44.28 (kJ)/(kg*K))
(b) air as an ideal gas ((Q)/(m)= -153.81 (kJ)/(kg*K); (W)/(m)= -153.81 (kJ)/(kg*K))
For each case (a)/(b), sketch the process on p-v and T-s coordinates.
Tips:
For both problems: Calculate the heat transfer per unit mass as a function of
entropy change; setup the energy balance