Home /
Expert Answers /
Chemical Engineering /
q-1-the-behavior-of-real-gasses-does-not-obey-the-one-of-the-ideal-gases-per-pa679
(Solved): Q.1 The behavior of real gasses does not obey the one of the ideal gases (per ...
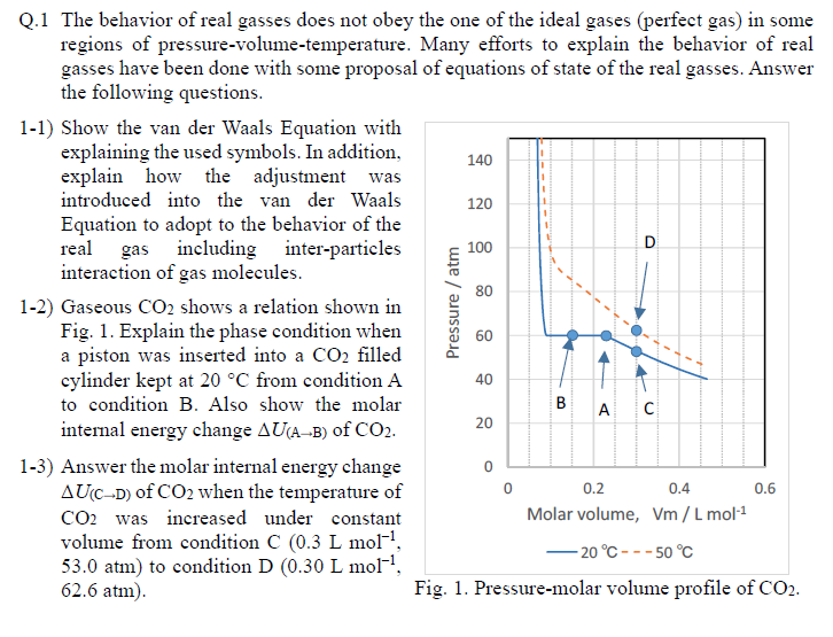
Q.1 The behavior of real gasses does not obey the one of the ideal gases (perfect gas) in some regions of pressure-volume-temperature. Many efforts to explain the behavior of real gasses have been done with some proposal of equations of state of the real gasses. Answer the following questions. 1-1) Show the van der Waals Equation with explaining the used symbols. In addition, explain how the adjustment was introduced into the van der Waals Equation to adopt to the behavior of the real gas including inter-particles interaction of gas molecules. 1-2) Gaseous shows a relation shown in Fig. 1. Explain the phase condition when a piston was inserted into a filled cylinder kept at from condition to condition . Also show the molar internal energy change of . 1-3) Answer the molar internal energy change of when the temperature of was increased under constant volume from condition , to condition , . Fig. 1. Pressure-molar volume profile of .
Expert Answer
The van der Waals equation is an equation of state for real gases that accounts for inter-particle interactions and deviations from ideal gas behavior. It is expressed as: Where,P = pressure of the gasV = volume of the gasn = number of moles of the gasR = gas constantT = temperature of the gasa and b are the van der Waals constants, which are specific to each gas.The adjustment introduced into the van der Waals equation to adopt to the behavior of real gases including inter-particles interaction of gas molecules is as follows:The term (n/V)^2 represents the effect of molecular volume, which reduces the available volume for movement of gas molecules.The term nb adjusts the volume of the container to account for the space occupied by the gas molecules.The constant a adjusts for the attractive forces between gas molecules, which reduce the pressure observed.The constant b adjusts for the repulsive forces between gas molecules at close distances, which reduce the volume available for movement of gas molecules.