Home /
Expert Answers /
Chemical Engineering /
q-2-answer-the-following-questions-2-1-reaction-of-a-was-investigated-with-pa590
(Solved): Q.2 Answer the following questions. 2-1) Reaction of A was investigated with ...
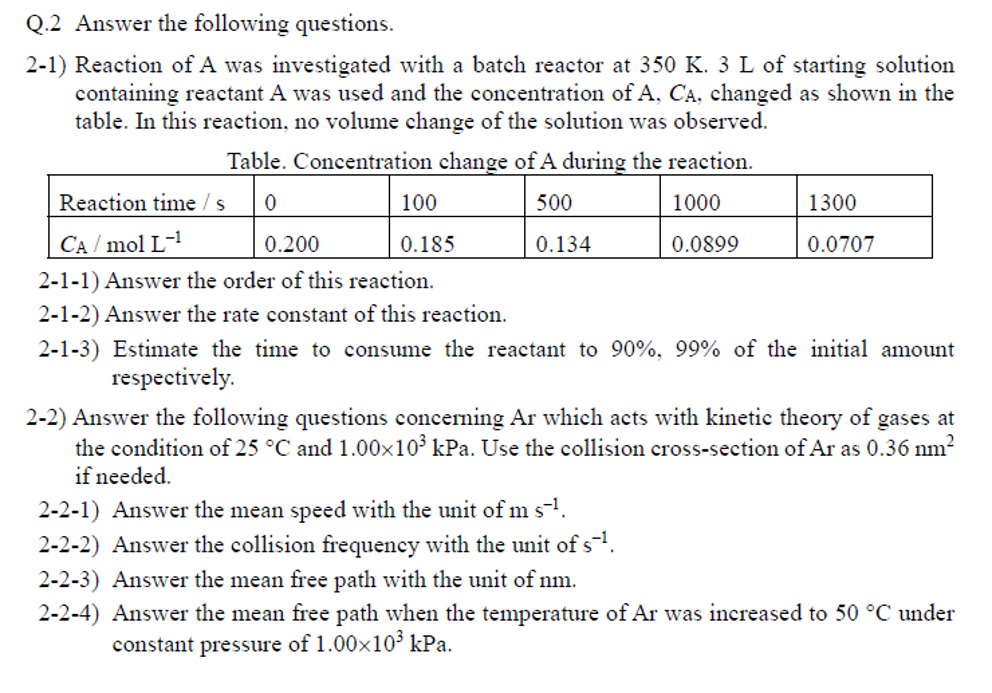
Q.2 Answer the following questions. 2-1) Reaction of was investigated with a batch reactor at of starting solution containing reactant was used and the concentration of , changed as shown in the table. In this reaction, no volume change of the solution was observed. Table. Concentration change of A during the reaction. 2-1-1) Answer the order of this reaction. 2-1-2) Answer the rate constant of this reaction. 2-1-3) Estimate the time to consume the reactant to of the initial amount respectively. 2-2) Answer the following questions concerning Ar which acts with kinetic theory of gases at the condition of and . Use the collision cross-section of Ar as if needed. 2-2-1) Answer the mean speed with the unit of . 2-2-2) Answer the collision frequency with the unit of . 2-2-3) Answer the mean free path with the unit of nm. 2-2-4) Answer the mean free path when the temperature of was increased to under constant pressure of .
Expert Answer
2-1-1) To determine the order of the reaction, we need to calculate the reaction rate constant for each data set and check whether the rate is constant or not. Using the data provided, we can calculate the rate constant for each interval using the formula: where CA is the concentration of A and t is the time.The order of the reaction can be determined by analyzing the relationship between k and CA. As we can see from the table, the rate constant remains constant throughout the reaction, indicating that the reaction is a first-order reaction.Therefore, the order of the reaction is 1st order.