Home /
Expert Answers /
Chemistry /
q-3b-and-5-please-3-calculate-the-mathrm-ph-values-of-the-following-water-samples-a-a-so-pa887
(Solved): Q 3b and 5 please 3. Calculate the \( \mathrm{pH} \) values of the following water samples: a. A so ...
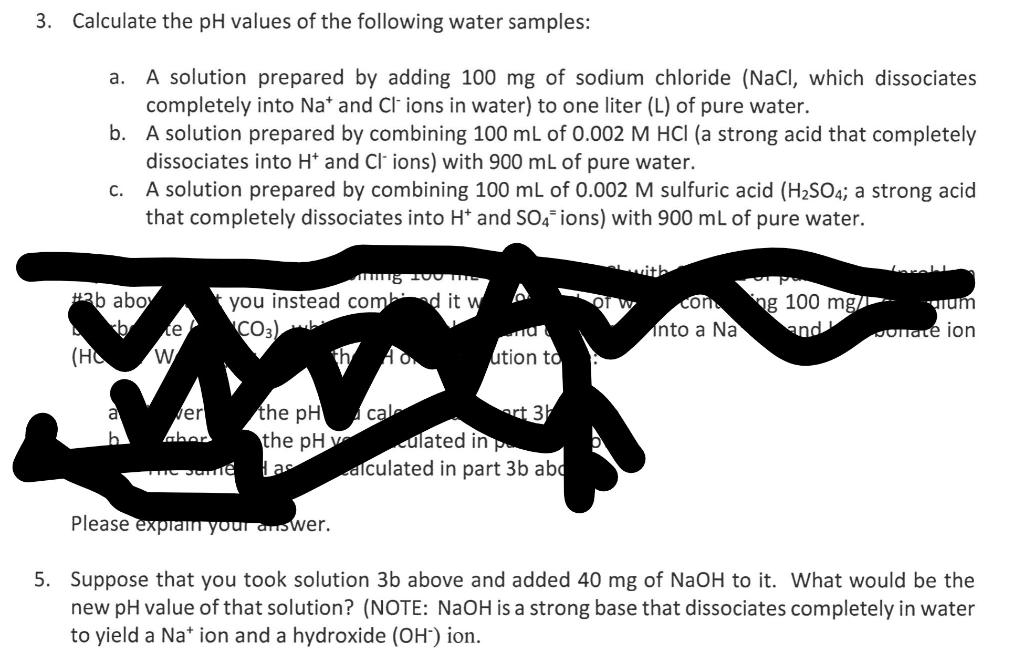
3. Calculate the \( \mathrm{pH} \) values of the following water samples: a. A solution prepared by adding \( 100 \mathrm{mg} \) of sodium chloride \( (\mathrm{NaCl} \), which dissociates completely into \( \mathrm{Na}^{+} \)and \( \mathrm{Cl}^{-} \)ions in water) to one liter ( \( \mathrm{L} \) ) of pure water. b. A solution prepared by combining \( 100 \mathrm{~mL} \) of \( 0.002 \mathrm{M} \mathrm{HCl} \) (a strong acid that completely dissociates into \( \mathrm{H}^{+} \)and \( \mathrm{Cl}^{-} \)ions) with \( 900 \mathrm{~mL} \) of pure water. c. A solution prepared by combining \( 100 \mathrm{~mL} \) of \( 0.002 \mathrm{M} \) sulfuric acid \( \left(\mathrm{H}_{2} \mathrm{SO}_{4}\right. \); a strong acid that completely dissociates into \( \mathrm{H}^{+} \)and \( \mathrm{SO}_{4}= \) ions) with \( 900 \mathrm{~mL} \) of pure water. Suppose that you took solution \( 3 \mathrm{~b} \) above and added \( 40 \mathrm{mg} \) of \( \mathrm{NaOH} \) to it. What would be the new \( \mathrm{pH} \) value of that solution? (NOTE: \( \mathrm{NaOH} \) is a strong base that dissociates completely in water to yield a \( \mathrm{Na}^{+} \)ion and a hydroxide \( \left(\mathrm{OH}^{-}\right) \)ion.