Home /
Expert Answers /
Chemistry /
question-1-1-at-the-low-concentration-limit-does-sds-behave-as-a-strong-electrolyte-1-5-pts-2-pa743
(Solved): question 1 1. At the low concentration limit, does SDS behave as a strong electrolyte? (1.5 pts) 2. ...
question 1
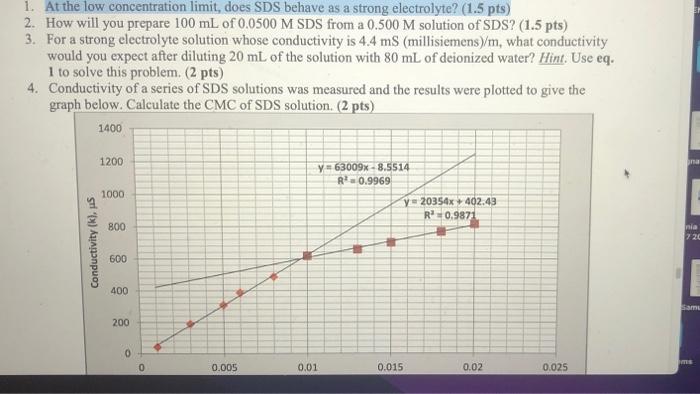

1. At the low concentration limit, does SDS behave as a strong electrolyte? (1.5 pts) 2. How will you prepare \( 100 \mathrm{~mL} \) of \( 0.0500 \mathrm{M} \) SDS from a \( 0.500 \mathrm{M} \) solution of SDS? (1.5 pts) 3. For a strong electrolyte solution whose conductivity is \( 4.4 \mathrm{mS} \) (millisiemens)/ \( \mathrm{m} \), what conductivity would you expect after diluting \( 20 \mathrm{~mL} \) of the solution with \( 80 \mathrm{~mL} \) of deionized water? Hint. Use eq. 1 to solve this problem. ( 2 pts) 4. Conductivity of a series of SDS solutions was measured and the results were plotted to give the graph below. Calculate the CMC of SDS solution. ( 2 pts)
Expert Answer
Sodium Dodecyl Sulfate is also known as SDS. It is the most frequent ionic surfactant. SDS is prepared as either a 10%