Home /
Expert Answers /
Chemistry /
question-1-use-the-standard-molar-entropies-given-in-each-part-to-calculate-s-for-pa723
(Solved): Question 1 Use the standard molar entropies given in each part to calculate S for ...


Question 1 Use the standard molar entropies given in each part to calculate for each of the following reactions. a) Enter an integer or dacimal number [morew]. b) c)
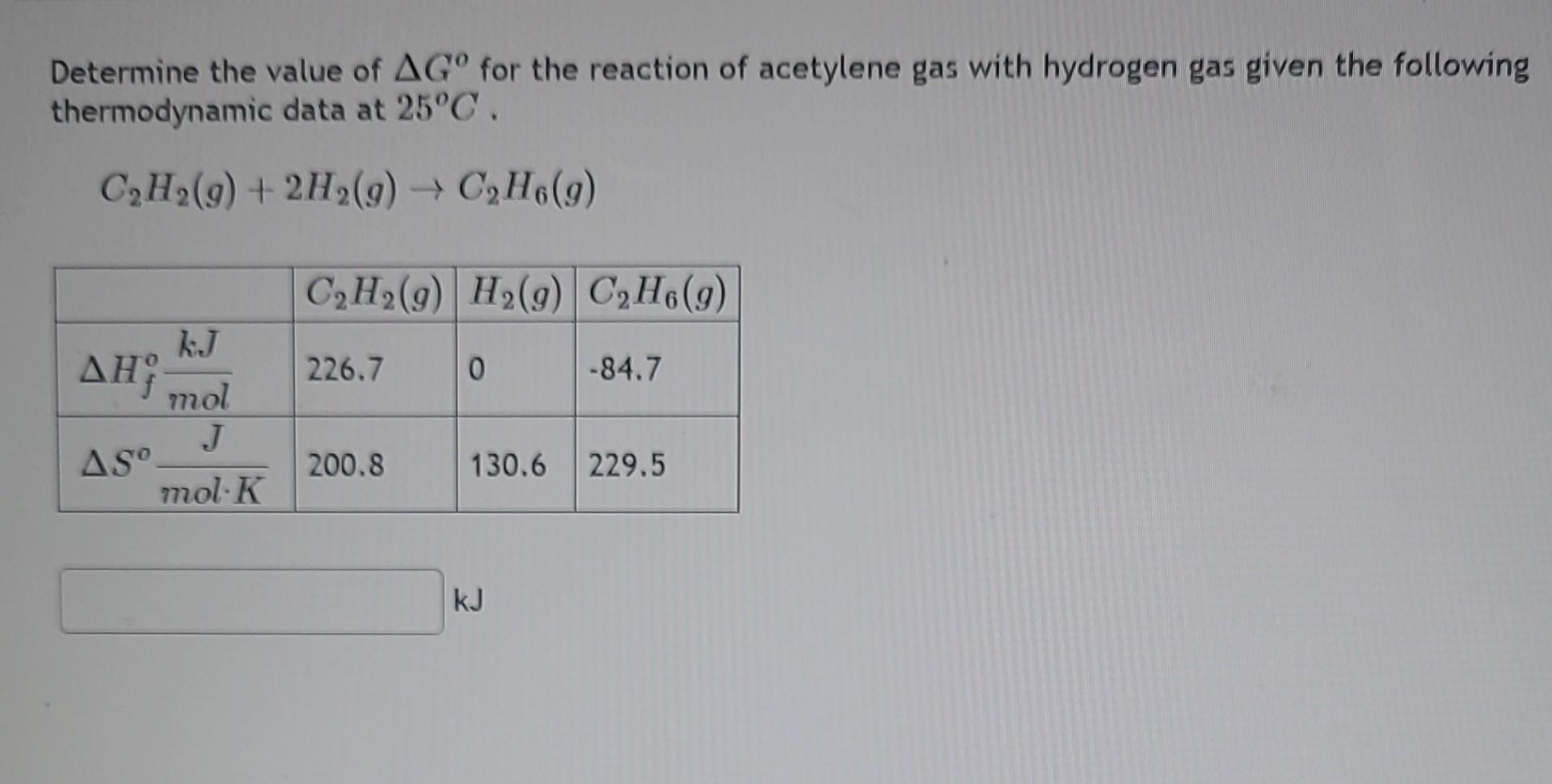
Determine the value of for the reaction of acetylene gas with hydrogen gas given the following thermodynamic data at .
Which of the following reactions is spontaneous only at low temperatures; only at high temperatures; at temperatures? Use the data table below to help calculate or as indicated to helf you determine the answer. a) The reaction is spontaneous at high temperatures all temperatures low temperatures b) The reaction is spontaneous at