Home /
Expert Answers /
Chemistry /
question-2-1-pts-given-the-following-redox-reactions-and-cell-potentials-determine-the-relative-st-pa281
(Solved): Question 2 1 pts Given the following redox reactions and cell potentials, determine the relative st ...

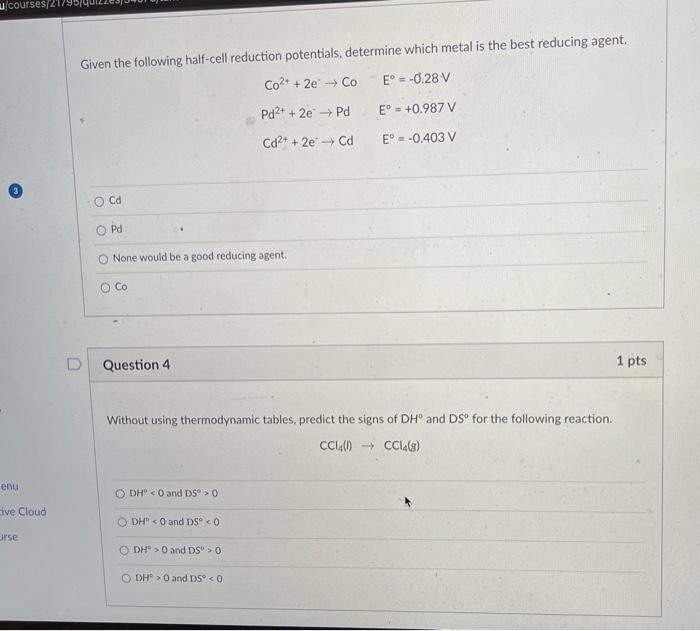
Question 2 1 pts Given the following redox reactions and cell potentials, determine the relative strengths of Al, Fe, and Cras reducing agents. (listed from weakest to strongest reducing agent) Al(s) + Cr3+ (aq) ? Al3+ (aq) + Cr(s) E = +0.966 V Fe(s) + Cr3+ (aq) ? Fe3+ (aq) + Cr(s) E° = -0.70 V -> Al 0 Live Cloud ODH° 0 and DS < 0 urse DH'>O and DS'>0 DH > O and DSP < 0