Home /
Expert Answers /
Chemistry /
question-3-cationic-polymerization-9-p-a-draw-the-structures-of-isotactic-syndiotactic-and-ata-pa252
(Solved): Question 3. Cationic Polymerization. (9 P) a) Draw the structures of isotactic, syndiotactic and ata ...
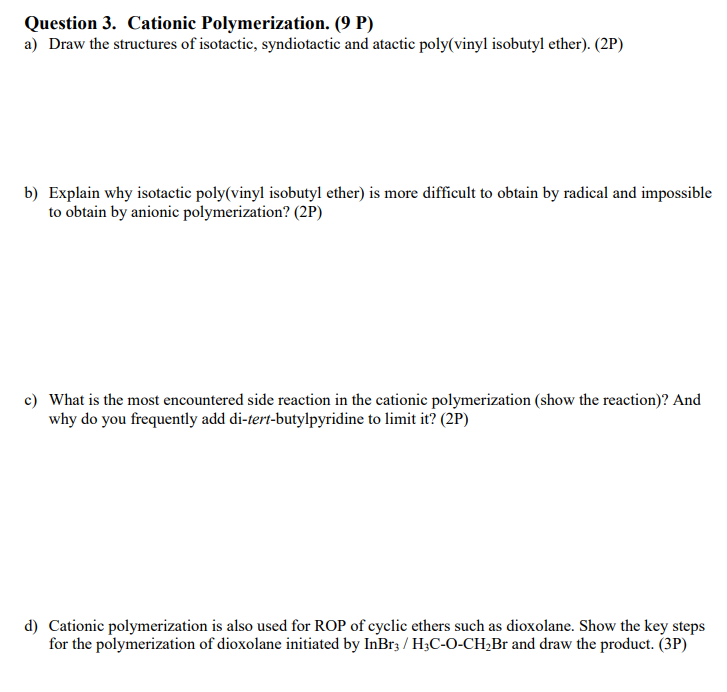
Question 3. Cationic Polymerization. (9 P) a) Draw the structures of isotactic, syndiotactic and atactic poly(vinyl isobutyl ether). (2P) b) Explain why isotactic poly(vinyl isobutyl ether) is more difficult to obtain by radical and impossible to obtain by anionic polymerization? (2P) c) What is the most encountered side reaction in the cationic polymerization (show the reaction)? And why do you frequently add di-tert-butylpyridine to limit it? (2P) d) Cationic polymerization is also used for ROP of cyclic ethers such as dioxolane. Show the key steps for the polymerization of dioxolane initiated by \( \mathrm{InBr}_{3} / \mathrm{H}_{3} \mathrm{C}-\mathrm{O}-\mathrm{CH}_{2} \mathrm{Br} \) and draw the product. (3P)