Home /
Expert Answers /
Chemistry /
question-3-not-yet-answered-points-out-of-600-remove-flag-a-buffer-is-made-up-of-0-582-m-in-nh-3-a-pa484
(Solved): Question 3 Not yet answered Points out of 600 Remove flag A buffer is made up of 0.582 M in NH_(3) a ...
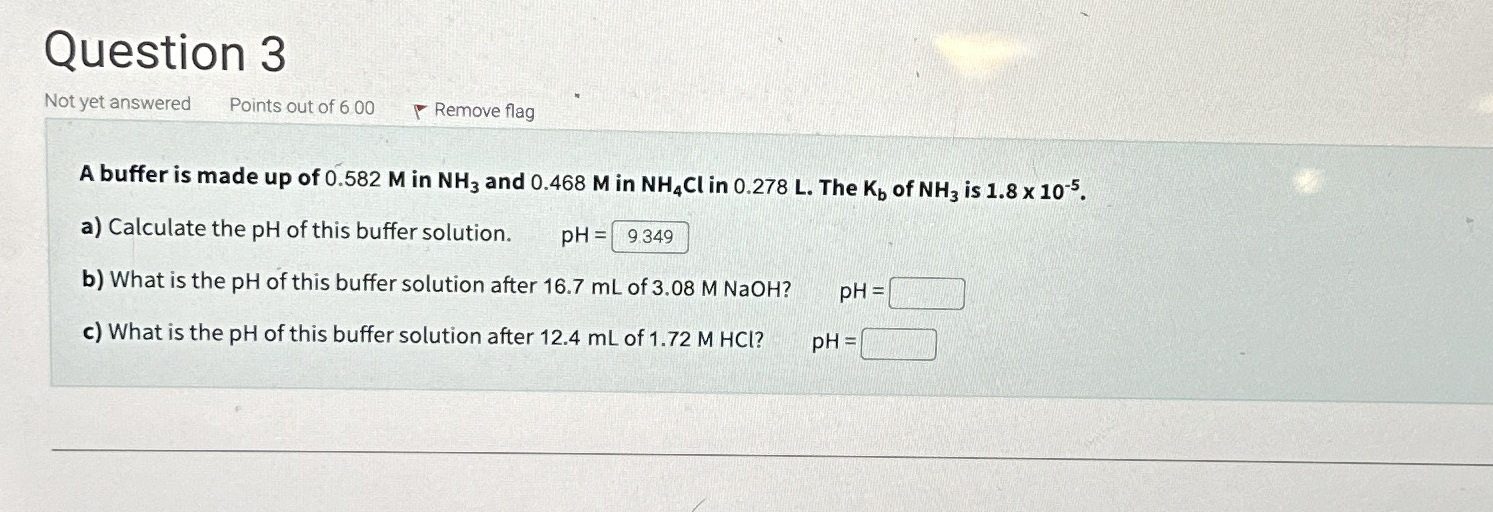
Question 3 Not yet answered Points out of 600 Remove flag A buffer is made up of 0.582 M in
NH_(3)and 0.468 M in
NH_(4)Clin 0.278 L . The
K_(b)of
NH_(3)is
1.8\times 10^(-5). a) Calculate the pH of this buffer solution.
pH=b) What is the pH of this buffer solution after 16.7 mL of 3.08 M NaOH ?
pH=c) What is the pH of this buffer solution after 12.4 mL of 1.72 M HCl ?
pH=