Home /
Expert Answers /
Chemistry /
question-6-the-last-step-scaling-and-adding-the-two-1-2-reactions-consider-the-following-oxidatio-pa847
(Solved): QUESTION 6 THE LAST STEP (scaling and adding the two 1/2 reactions) Consider the following oxidatio ...
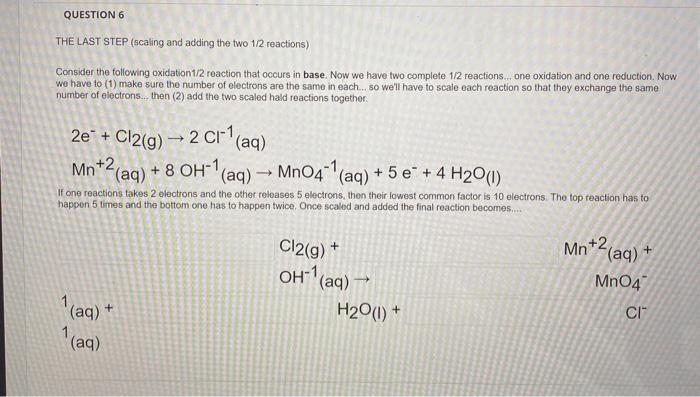
QUESTION 6 THE LAST STEP (scaling and adding the two 1/2 reactions) Consider the following oxidation 1/2 reaction that occurs in base. Now we have two complete 1/2 reactions... one oxidation and one reduction. Now we have to (1) make sure the number of electrons are the same in each so we'll have to scalo each reaction so that they exchange the same number of electrons...then (2) add the two scaled hald reactions together - 2e* + Cl2(g) – 2 C1 (aq) Mn +2(aq) + 8 OH-1 "(aq) -- Mn04-' (aq) + 5 e* + 4 H20(1) - If one reactions takes 2 electrons and the other releases 5 electrons, then their lowest common factor is 10 electrons. The top reaction has to happen 5 times and the bottom one has to happen twice. Once scaled and added the final reaction becomes... Cl2(g) + + OH(aq) ? Mn+2 (aq) + +2 MnO4 cl (aq) + 1(aq) H2O(0) +