Home /
Expert Answers /
Chemistry /
question-8-15-pts-in-this-experiment-6mhcl-aq-is-added-to-a-solution-to-lower-its-ph-to-pa851
(Solved): Question 8 - [15 pts] - In this experiment, 6MHCl_() (aq.) is added to a solution to lower its pH to ...
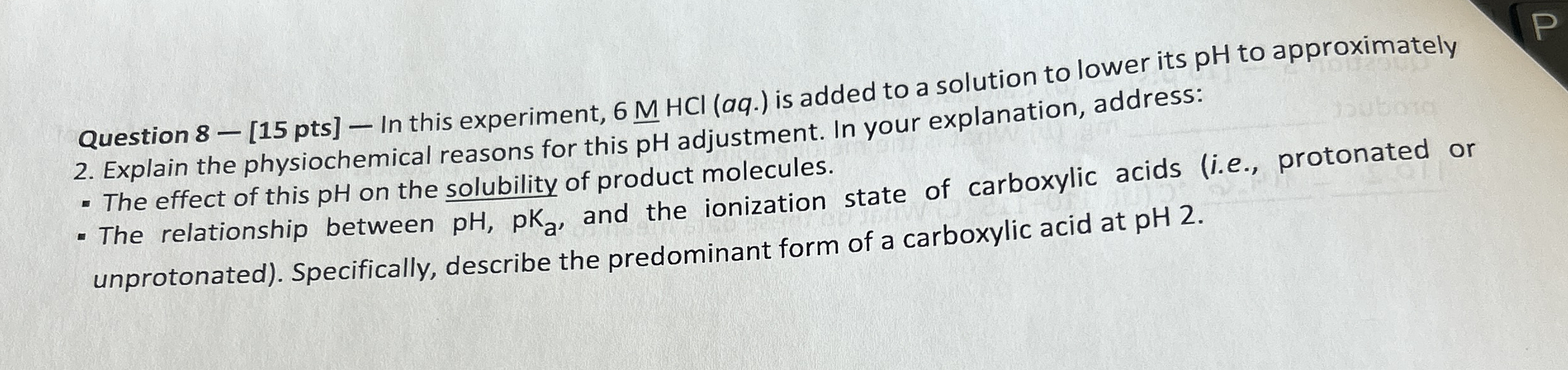
Question 8 - [15 pts] - In this experiment,
6MHCl_()(aq.) is added to a solution to lower its pH to approximately 2. Explain the physiochemical reasons for this pH adjustment. In your explanation, address: The effect of this pH on the solubility of product molecules. The relationship between
pH,pK_(a), and the ionization state of carboxylic acids (i.e., protonated or unprotonated). Specifically, describe the predominant form of a carboxylic acid at pH 2.