Home /
Expert Answers /
Chemistry /
question-8-mixing-things-up-final-exam-question-an-isolated-container-is-partitioned-in-two-compa-pa830
(Solved): Question 8. Mixing things up, final exam question An isolated container is partitioned in two compa ...
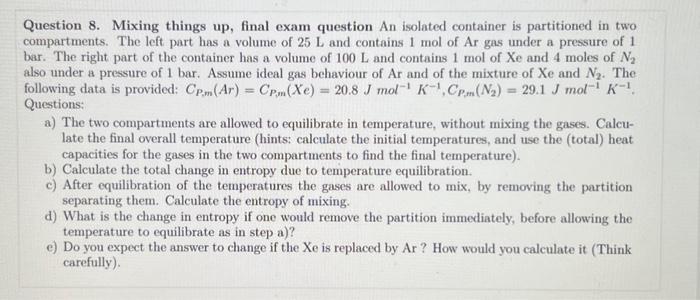
Question 8. Mixing things up, final exam question An isolated container is partitioned in two compartments. The left part has a volume of and contains 1 mol of Ar gas under a pressure of 1 bar. The right part of the container has a volume of and contains 1 mol of and 4 moles of also under a pressure of 1 bar. Assume ideal gas behaviour of Ar and of the mixture of and . The Questions: a) The two compartments are allowed to equilibrate in temperature, without mixing the gases. Calculate the final overall temperature (hints: calculate the initial temperatures, and use the (total) heat capacities for the gases in the two compartments to find the final temperature). b) Calculate the total change in entropy due to temperature equilibration. c) After equilibration of the temperatures the gases are allowed to mix, by removing the partition separating them. Calculate the entropy of mixing. d) What is the change in entropy if one would remove the partition immediately, before allowing the temperature to equilibrate as in step a)? e) Do you expect the answer to change if the Xe is replaced by Ar? How would you calculate it (Think carefully).
Expert Answer
a) To calculate the final overall temperature, we can use the principle of energy conservation. The ...