Home /
Expert Answers /
Biology /
questions-a-through-p-0-the-free-energy-diagram-for-the-conversion-of-compound-x-to-compou-pa458
(Solved): Questions A through P. 0 The free energy diagram for the conversion of compound \( X \) to compou ...
Questions A through P.
0
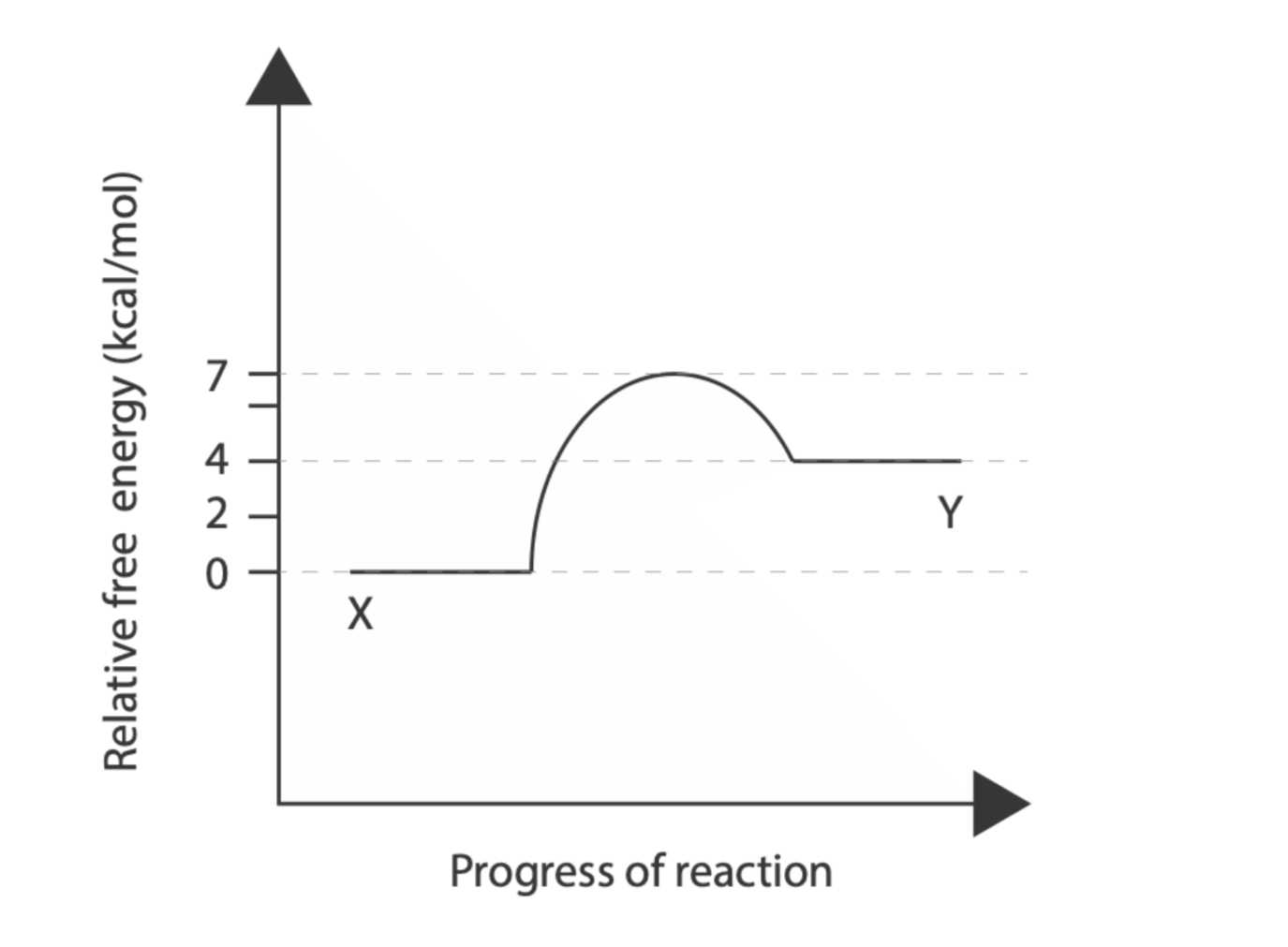
The free energy diagram for the conversion of compound \( X \) to compound \( Y \) is shown above. The following questions all relate to this reaction and/or free energy diagram. 16 sub-questions, each worth \( 0.25 \mathrm{pt} \) for \( 4 \mathrm{pt} \) total (a) What is the free energy change of the forward reaction? (b) What is the free energy change of the reverse reaction? (c) What is the activation energy for the forward reaction? (d) What is the activation energy for the reverse reaction? (e) Which reaction (forward or reverse) would need to be coupled to another reaction to proceed in the cell? (f) The free energy change of ATP hydrolysis is \( -7.3 \mathrm{kcal} \).mol: could hydrolysis of one ATP molecule be used to drive the conversion of one molecule of this reaction? If we add an enzyme (imaginatively named XYase), how will the following compare to the uncatalyzed reaction (answer using a single word: 'increases', 'decreases', or 'unchanged')? (g) Free energy change of the forward reaction (h) Free energy change of the reverse reaction (i) Activation energy of the forward reaction (j) Activation energy of the reverse reaction You add a very small amount of the the XYase enzyme, purified from the bacterium E. coli, to a tube containing a large excess of molecule \( Y \) (such that there are initially ten thousand times as many molecules of \( \mathrm{Y} \) as of \( \mathrm{XYase} \) ). At \( 37^{\circ} \mathrm{C} \), one molecule of enzyme can catalyze the conversion of 100 molecules of \( \mathrm{Y} \) per second, generating 10 molecules of \( X \). The uncatalyzed reaction proceeds much more slowly at all temperatures. Compared to the amount of \( X \) after 10 seconds at \( 37^{\circ} \mathrm{C} \), how will the amount of \( \mathrm{X} \) change under the following conditions (answer using a single word: 'increases', 'decreases', or 'unchanged')? (k) Putting the tube on ice \( \left(0^{\circ} \mathrm{C}\right) \) ? (I) Putting the tube at \( 95^{\circ} \mathrm{C} \) ? (m) Adding a variant of \( X \) Yase that binds much more tightly to \( X \) (doesn't release product) (n) Adding a variant of \( X \) Yase that binds much more weakly to \( Y \) (doesn't bind substrate) (o) Adding a competitive inhibitor of the enzyme (p) Adding an allosteric inhibitor of the enzyme
Expert Answer
For the purpose of understanding phases and phase