Home /
Expert Answers /
Chemistry /
report-table-sec-1-polarity-of-solutes-and-solvents-table-view-list-view-solubility-and-polarity-pa882
(Solved): Report Table SEC.1: Polarity of Solutes and Solvents Table view List view Solubility and polarity ...
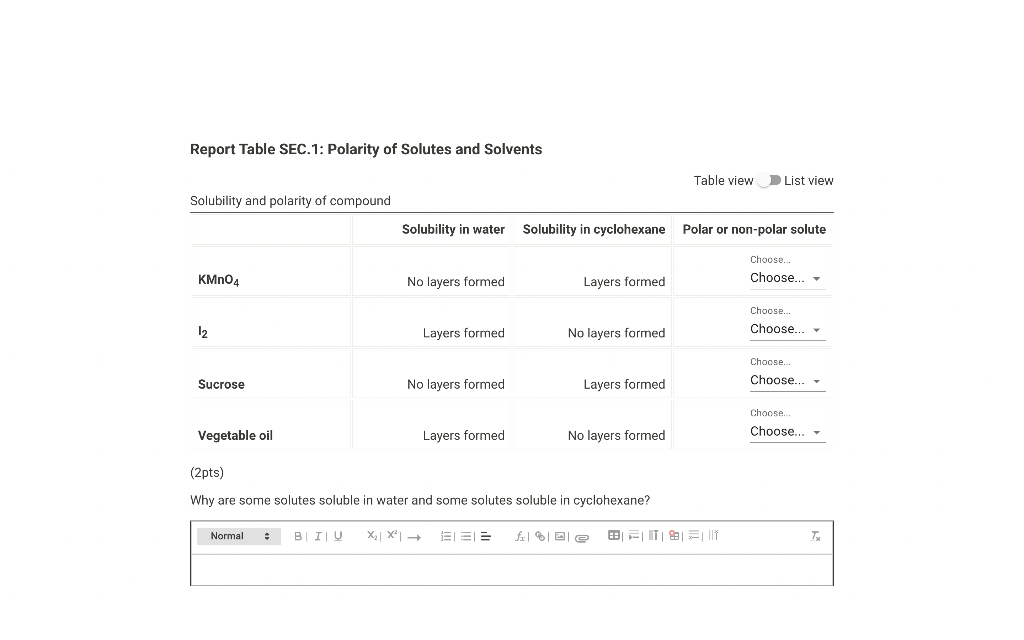
Report Table SEC.1: Polarity of Solutes and Solvents Table view List view Solubility and polarity of compound (2pts) Why are some solutes soluble in water and some solutes soluble in cyclohexane?
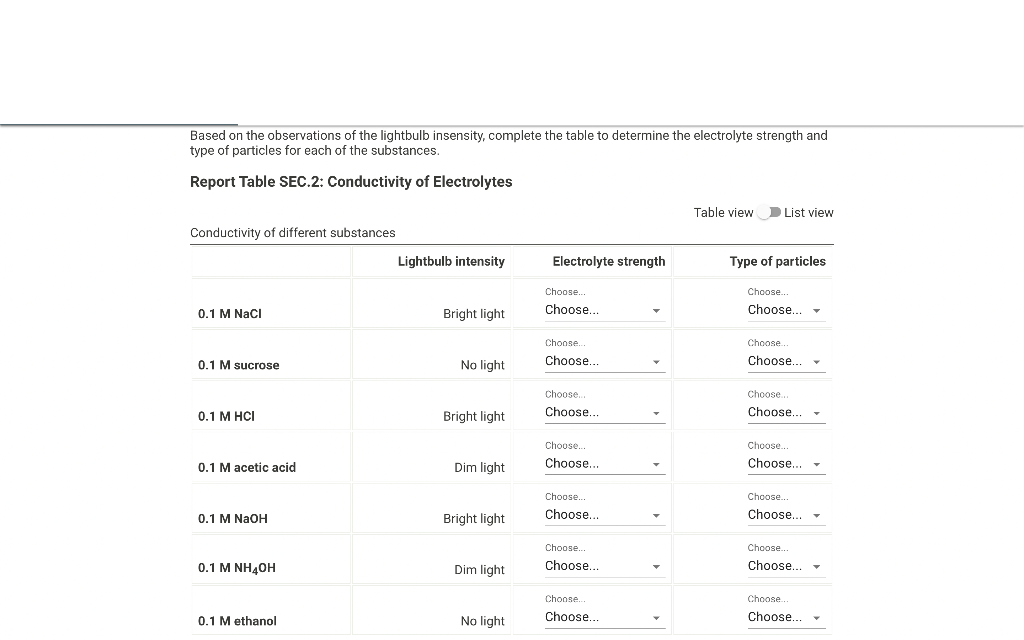
Based on the observations of the lightbulb insensity, complete the table to determine the electrolyte strength and type of particles for each of the substances. Report Table SEC.2: Conductivity of Electrolytes Table view List view Conductivitv of different substances
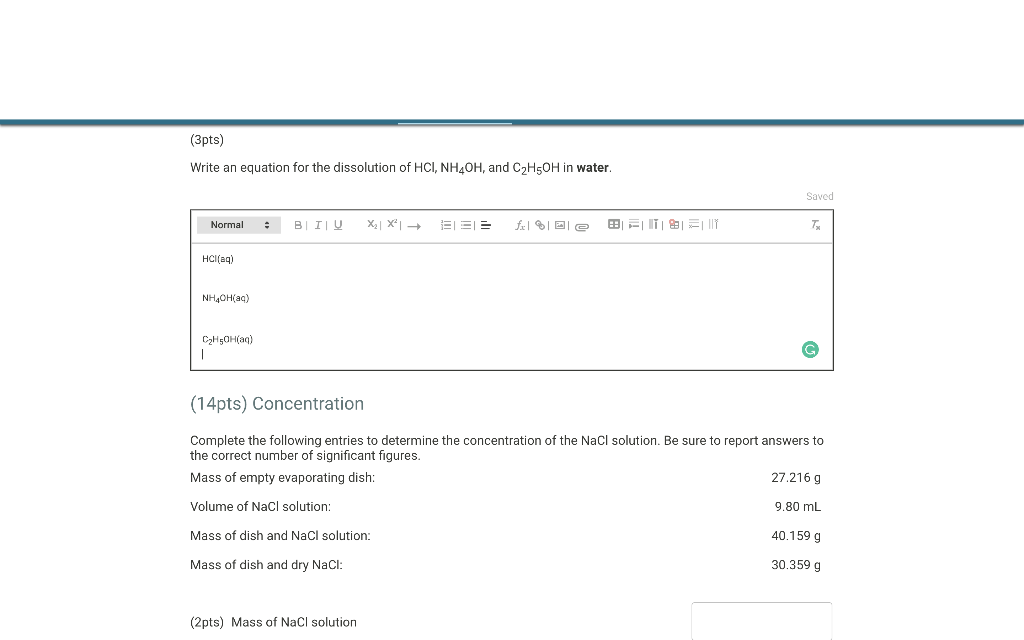
(3pts) Write an equation for the dissolution of \( \mathrm{HCl}, \mathrm{NH}_{4} \mathrm{OH} \), and \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \) in water. (14pts) Concentration Complete the following entries to determine the concentration of the \( \mathrm{NaCl} \) solution. Be sure to report answers to the correct number of significant figures. Mass of empty evaporating dish: \( 27.216 \mathrm{~g} \) Volume of \( \mathrm{NaCl} \) solution: \( 9.80 \mathrm{~mL} \) Mass of dish and \( \mathrm{NaCl} \) solution: \( 40.159 \mathrm{~g} \) Mass of dish and dry \( \mathrm{NaCl} \) : \( 30.359 \mathrm{~g} \) (2pts) Mass of \( \mathrm{NaCl} \) solution
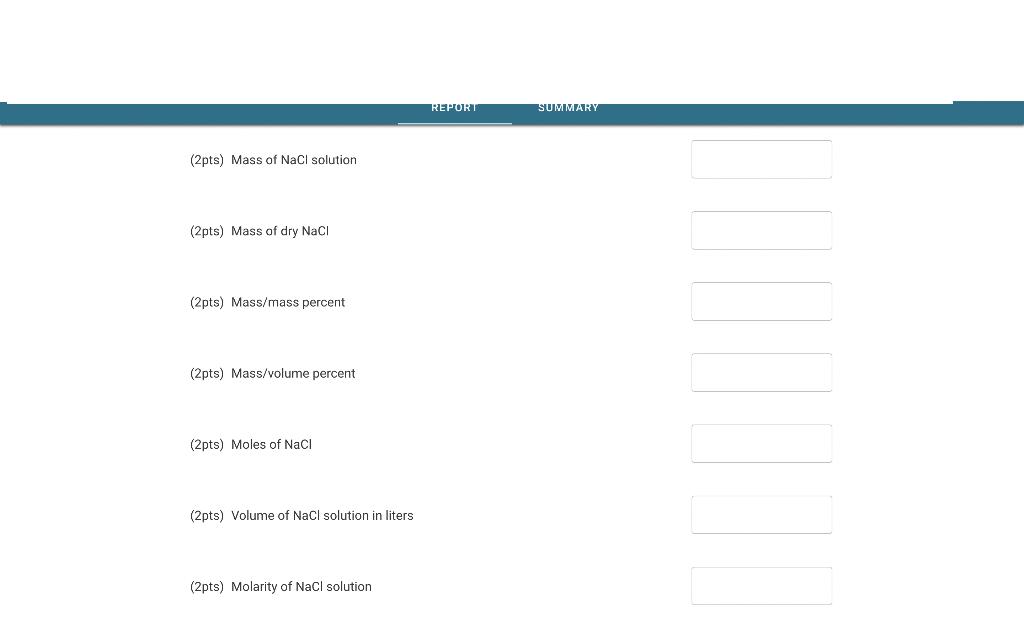
(2pts) Mass of \( \mathrm{NaCl} \) solution (2pts) Mass of dry \( \mathrm{NaCl} \) (2pts) Mass/mass percent (2pts) Mass/volume percent (2pts) Moles of \( \mathrm{NaCl} \) (2pts) Volume of \( \mathrm{NaCl} \) solution in liters (2pts) Molarity of \( \mathrm{NaCl} \) solution