Home /
Expert Answers /
Chemistry /
search-39-phet-39-and-39-molecules-and-light-39-run-the-simulation-select-39-ultraviolet-39-play-aro-pa341
(Solved): Search 'phet' and 'molecules and light'. Run the simulation. Select 'Ultraviolet'. Play around wit ...
Search 'phet' and 'molecules and light'. Run the simulation. Select 'Ultraviolet'. Play around with the different molecules and the lamp. Use the green button on the lamp to turn it on. How does ultraviolet radiation interact with each of the molecules in the list? What do you observe? For the molecules that show a change, write a reaction for the change you see.
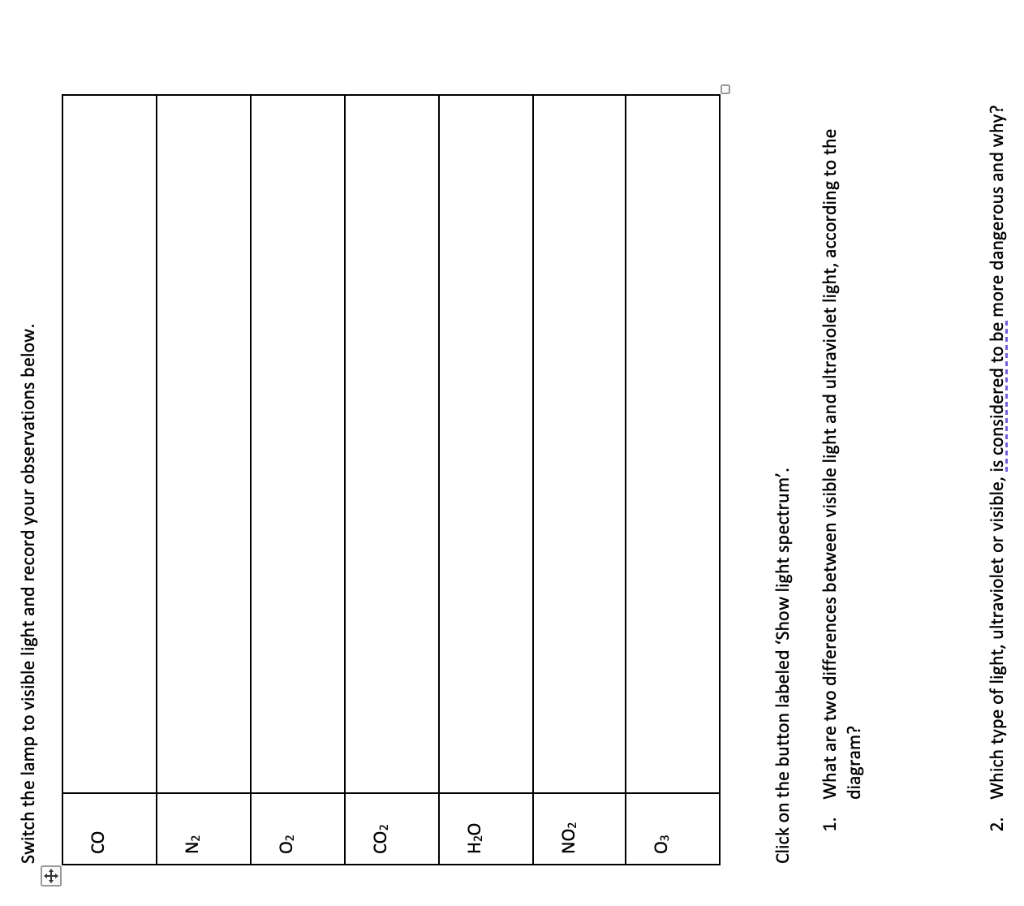
Switch the lamp to visible light and record your observations below. \begin{tabular}{|l|l|} \hline \( \mathrm{CO} \) & \\ \hline \( \mathrm{N}_{2} \) & \\ \hline \( \mathrm{O}_{2} \) & \\ \hline \( \mathrm{CO}_{2} \) & \\ \hline \( \mathrm{O}_{2} \mathrm{O} \) & \\ \hline \( \mathrm{NO}_{2} \) & \\ \hline \end{tabular} Click on the button labeled 'Show light spectrum'. 1. What are two differences between visible light and ultraviolet light, according to the diagram? 2. Which type of light, ultraviolet or visible, is considered to be more dangerous and why?
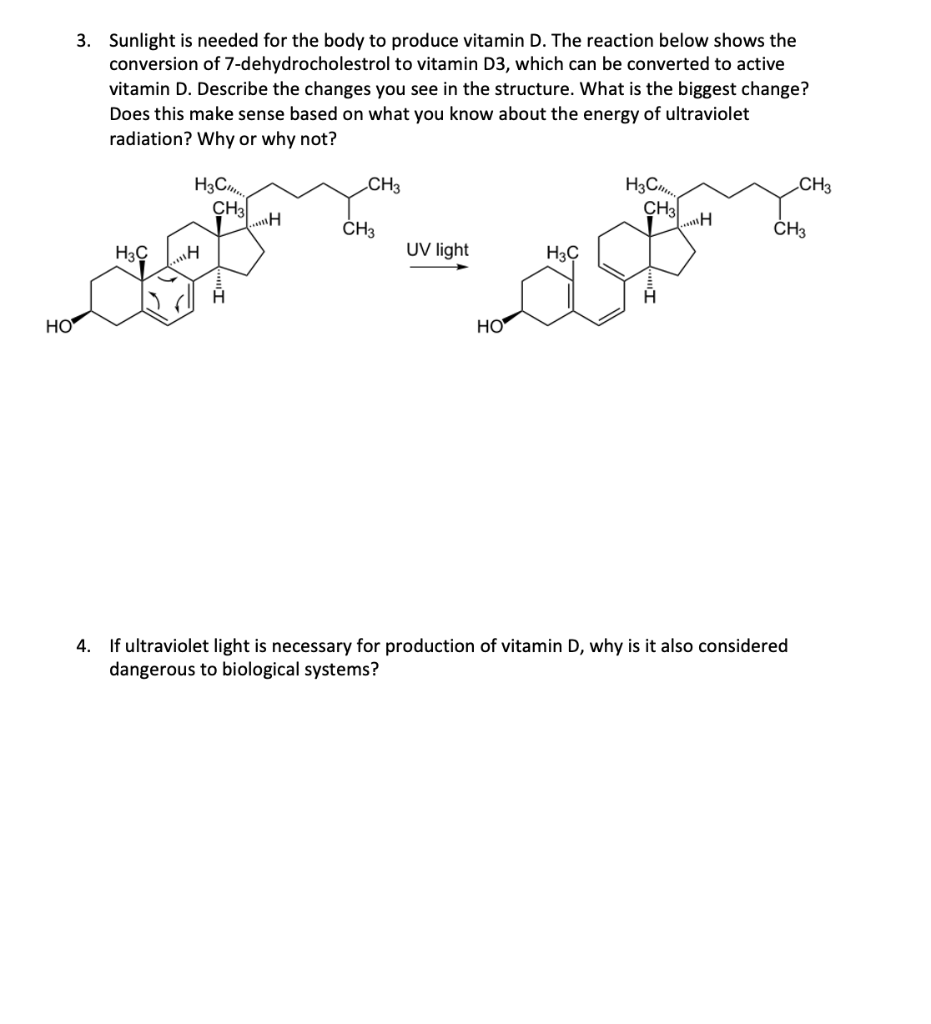
3. Sunlight is needed for the body to produce vitamin \( D \). The reaction below shows the conversion of 7-dehydrocholestrol to vitamin \( \mathrm{D} 3 \), which can be converted to active vitamin D. Describe the changes you see in the structure. What is the biggest change? Does this make sense based on what you know about the energy of ultraviolet radiation? Why or why not? \( \stackrel{\text { UV light }}{\longrightarrow} \) 4. If ultraviolet light is necessary for production of vitamin D, why is it also considered dangerous to biological systems?
5. Write out the steps of the Chapman cycle of ozone. Where is ultraviolet radiation involved? What does it mean when we say that ozone "absorbs" ultraviolet radiation? 6. The sun emits ultraviolet radiation, but where else can ultraviolet radiation come from? Search 'phet' and 'wave interference'. Run the simulation. Turn on the water (green button on the left side of the screen). When you increase and decrease the frequency, what happens? 7. When you increase and decrease the amplitude, what happens? 8. Click the third button under the amplitude scale to select the light source and turn on the light by clicking the green button. When you increase and decrease the frequency, what happens? How is it similar to water? How is it different? 9. Go to the bottom of the screen and click on the button labeled 'Interference'. Turn on both water spigots. What do you see where the waves overlap? 10. Switch to a light source by clicking on the light button (third over under the separation scale). Turn on both light sources. Are the patterns similar to what you saw for water? Do you expect them to be and why or why not?
11. Go to the bottom of the page and select 'Slits'. Turn on the water wave generator. Describe what happens to the water wave when it goes through the slit. 12. Switch to a light source by clicking on the third button under the Amplitude scale and turn on the light. What happens to the light wave when it goes through the slit? Is this the same as what you see for water and is this what you would expect? 13. Light is said to have wave-particle duality. What does this mean? 14. Based on the simulations, what is one example where you saw light behaving as a wave? What is one example where light can be described as a particle?
Expert Answer
3. The biggest change in the structure in the breaking of c-c single bond. Yes this makes sense because we know that light has lower wavelength than visible light and more energy than visible light. The energy more energy needed to break c-c bond is