Home /
Expert Answers /
Chemistry /
sigma-bonds-can-form-between-different-types-of-orbitals-the-main-criterion-for-sigma-bond-formati-pa796
(Solved): Sigma bonds can form between different types of orbitals. The main criterion for sigma bond formati ...

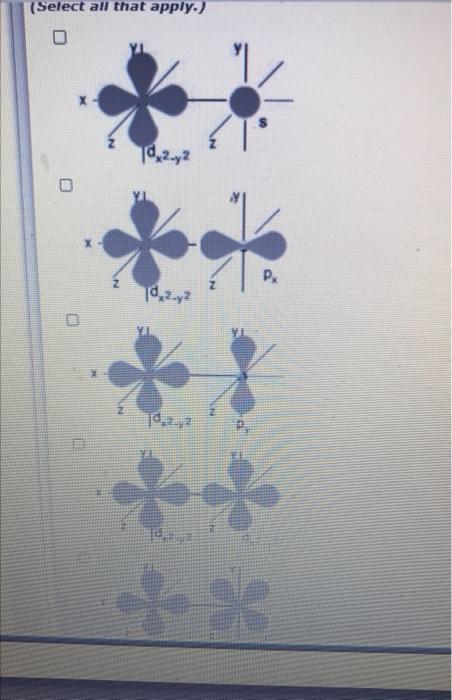
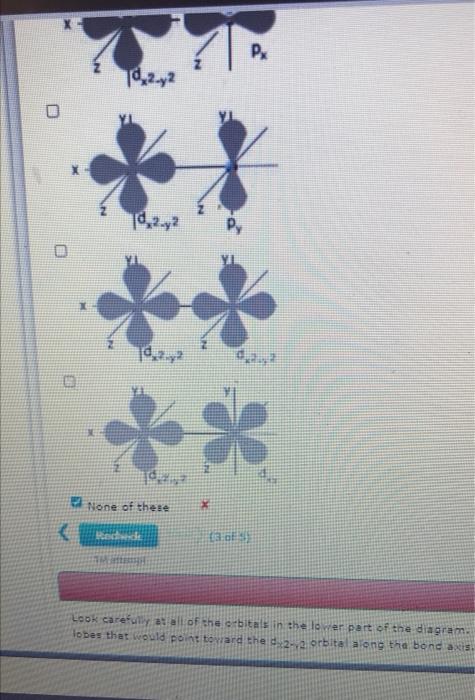
Sigma bonds can form between different types of orbitals. The main criterion for sigma bond formation is that the two bonded atoms have valence orbitals with lobes that point directly at each othwe along the line between the two nuclei. The images below show the shapes of two orbitals on adjacent atoms. Choose all of the combinations that will lead to formation of a sigma bond.
(Select all that apply.) 0 Td 2.2 X Pr 10,2.2 **
CA Py B Px ?² dzi None of these Redwede (3 of 5) FA Look carefully at all of the orbitals in the lower part of the diagram. lobes that would point toward the d2-2 orbital along the bond axis. ?jam?
Expert Answer
-: Answer :- - For the overlapping of the d(x2-y2) and S orbital there occurs in the unfavorable condition of the overlapping and hence there occurs in the formation of the antibonding orbital. Therefore there is no possibility of the formation of th