Home /
Expert Answers /
Chemistry /
the-caffeine-benzoic-acid-and-aspartame-content-in-a-commercial-beverage-was-analyzed-by-hplc-the-pa969
(Solved): The caffeine, benzoic acid and aspartame content in a commercial beverage was analyzed by HPLC. The ...
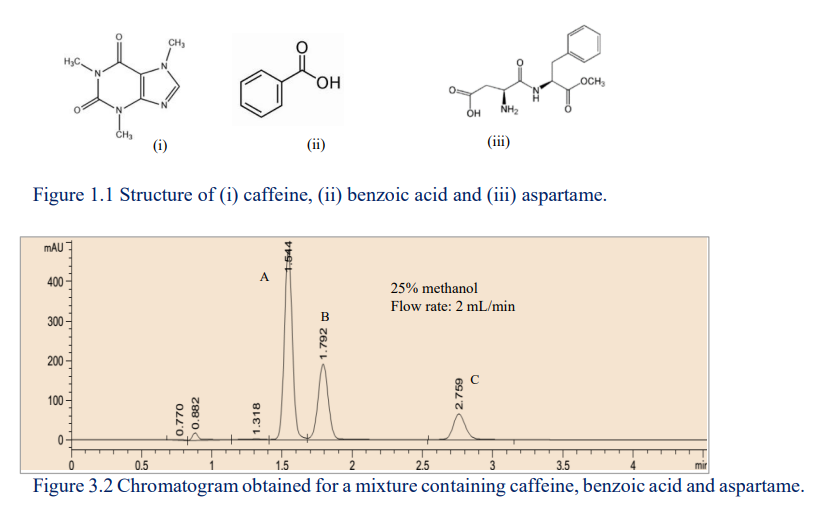
The caffeine, benzoic acid and aspartame content in a commercial beverage was analyzed by HPLC. The three analytes were identified as peaks A, B and C in the chromatograms shown below
???????
Question
a. Briefly explain why a normal-phase column is not suitable for separating these molecules.
b. Briefly discuss the role of methanol in the mobile phase.
c. Rank the polarity of the tree analytes. Justify your answer.
d. Identify the analytes that came out last with justification.
e. Calculate the Rs values between peaks A and B. Explain whether the separation of A from B is complete.
Figure 1.1 Structure of (i) caffeine, (ii) benzoic acid and (iii) aspartame.
Expert Answer
a) normal phase column or chromatography is not suitable for separation of ionic or polar compounds since benzoic acid, caffeine