Home /
Expert Answers /
Chemistry /
the-everape-humani-body-contains-6-40-mathrm-l-el-blood-wth-a-mathrm-fe-2-concen-pa460
(Solved): The Everape humani body contains \( 6.40 \mathrm{~L} \) el blood wth a \( \mathrm{Fe}^{2} \) concen ...
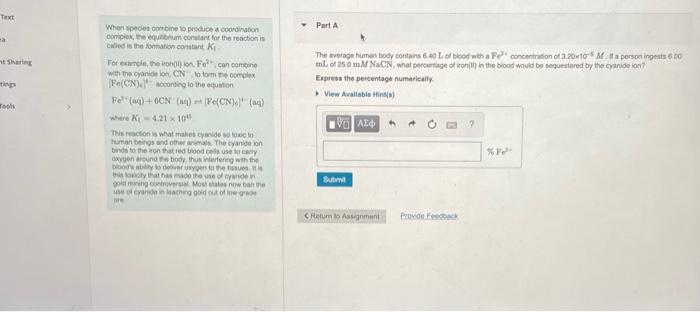
The Everape humani body contains \( 6.40 \mathrm{~L} \) el blood wth a \( \mathrm{Fe}^{2} \) concentradon of \( 3.20 \mathrm{n}^{-5} \). Af i a person ingests 660 For exancle, the boholion. Feit, can comeine tal of \( 25.0 \mathrm{~m} M \mathrm{NaCN} \), what persentage of iron(ii) in the biood would be sequestered by the cyanide ion? wth the cyaniolion, \( \mathrm{CN} \), lo form the complex \( \left[\mathrm{Fe}(\mathrm{CN})_{\mathrm{e}}\right]^{1-} \) according to the equation Express the pereentage numerically. \( \left.\mathrm{Pe}^{3}+(\mathrm{aqg})+6 \mathrm{CN}(\mathrm{aq})=[\mathrm{Pe}(\mathrm{CN})]^{4}(\mathrm{aq})\right] \) where \( K_{i}=4.21 \times 10^{4} \) This reacion is what mahes cymide so taxic to human beivos and other arimals. The cyande ion binds to the iron that ted bood cells use ia cely exyoer around tre booy, thus interfering with the thoods ablity be deicer usyuen ta the bssues. An in the kristy toat has made the use of cyanide in gota mineing Cyidryersw Moer otates now ban the