(Solved): The Fischer esterification mechanism is examined in this question. The overall reaction is: CH_(3)O ...
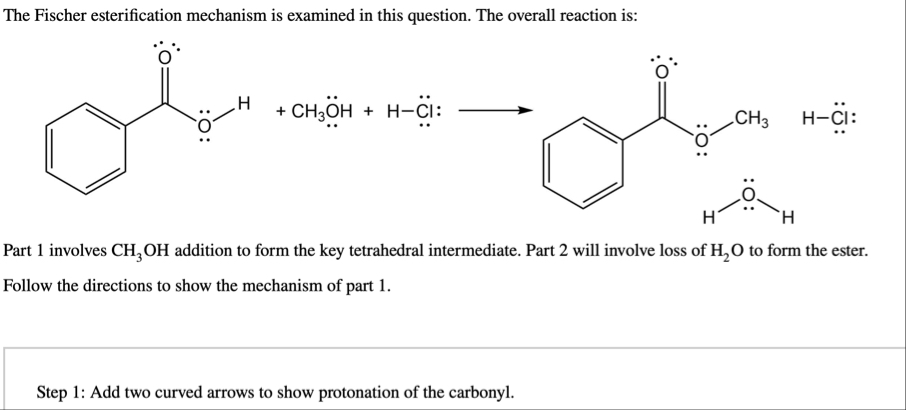
The Fischer esterification mechanism is examined in this question. The overall reaction is:
CH_(3)O^(¨)H H-Cl^(¨):longrightarrowPart 1 involves
CH_(3)OHaddition to form the key tetrahedral intermediate. Part 2 will involve loss of
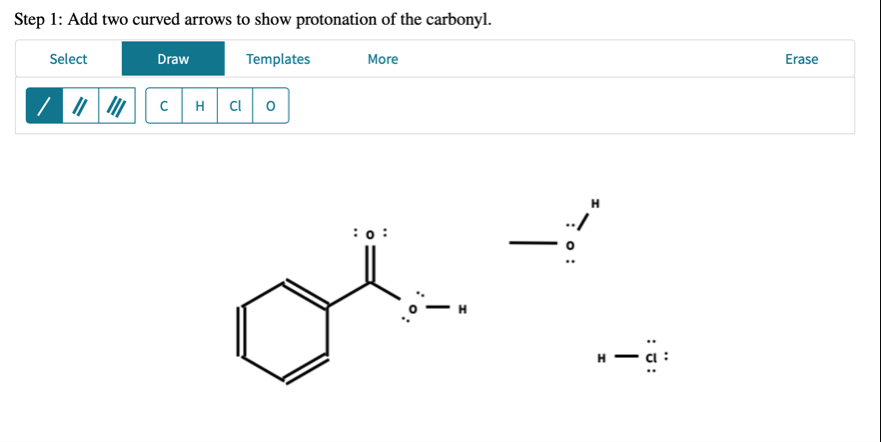
H_(2)Oto form the ester. Follow the directions to show the mechanism of part 1. Step 1: Add two curved arrows to show protonation of the carbonyl.Step 1: Add two curved arrows to show protonation of the carbonyl.
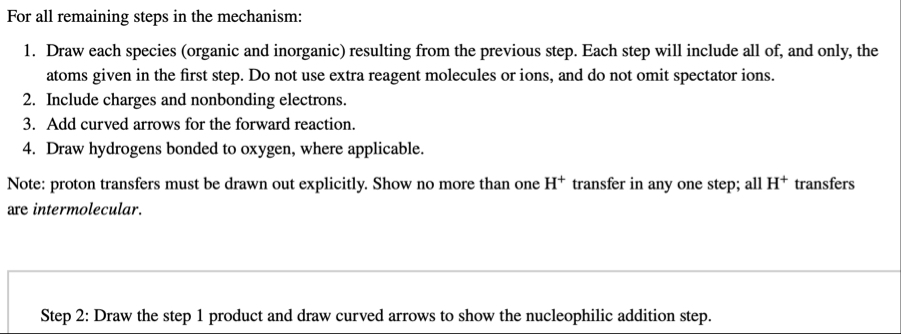
◻For all remaining steps in the mechanism: Draw each species (organic and inorganic) resulting from the previous step. Each step will include all of, and only, the atoms given in the first step. Do not use extra reagent molecules or ions, and do not omit spectator ions. Include charges and nonbonding electrons. Add curved arrows for the forward reaction. Draw hydrogens bonded to oxygen, where applicable. Note: proton transfers must be drawn out explicitly. Show no more than one
H^( )transfer in any one step; all
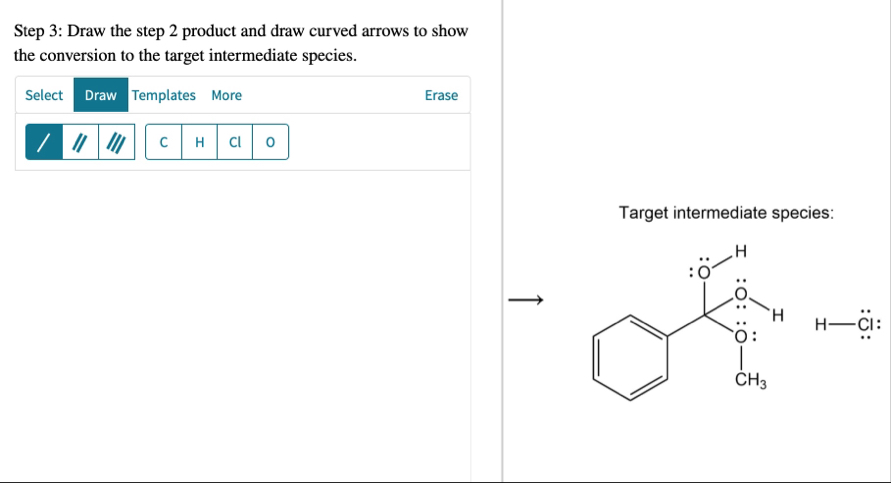
H^( )transfers are intermolecular. Step 2: Draw the step 1 product and draw curved arrows to show the nucleophilic addition step.Step 3: Draw the step 2 product and draw curved arrows to show the conversion to the target intermediate species.
◻Draw Templates More Erase ///I/I \table[[
C,H,Cl,O]]