(Solved): The following reaction illustrates the use of a cyano compound with an organolithium reagent. Draw a ...
The following reaction illustrates the use of a cyano compound with an organolithium reagent. Draw a curved arrow mechanism of the second step of this reaction. Show all intermediate structures.
◻Draw the expected major product of the following reduction.
◻
H_(2)OUsing the curved arrow formalism, draw a mechanism to show the following isomerization reaction. Draw the expected major product of the following reaction.
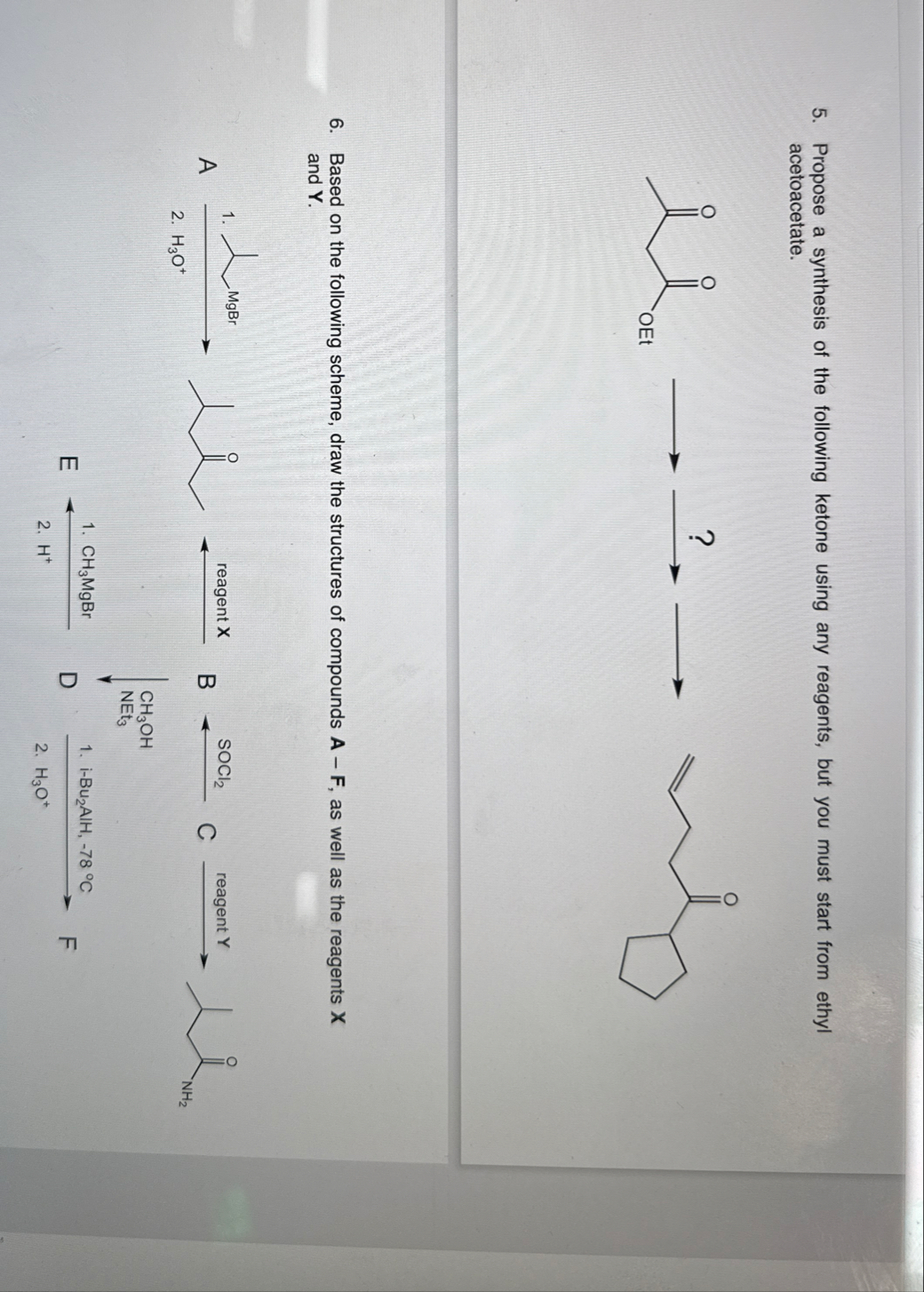
◻Propose a synthesis of the following ketone using any reagents, but you must start from ethyl acetoacetate. Based on the following scheme, draw the structures of compounds
A-F, as well as the reagents
xand
Y. Based on the following scheme, draw the structures of compounds
Hand
I.
->_(2.)^(1.LDA,-78^(@)C)->_(H_(2)O)^(H_(3)O^( ))HNaOH
H_(3)O^( )heat There are multiple methods to convert a primary amide to a nitrile. Your textbook (section 20.7) shows the mechanism for this conversion using thionyl chloride. Using the mechanism provided in the textbook as a guide, propose a mechanism for the same conversion using acetic anhydride (a carboxylic acid anhydride). Propose a synthesis of the following ketone using any reagents, but you must start from ethyl acetoacetate. Based on the following scheme, draw the structures of compounds
A-F, as well as the reagents
xand
Y.