(Solved): The homework solutions including the regarding MATLAB codes should be submitted by 05.01.2025, 23:59 ...
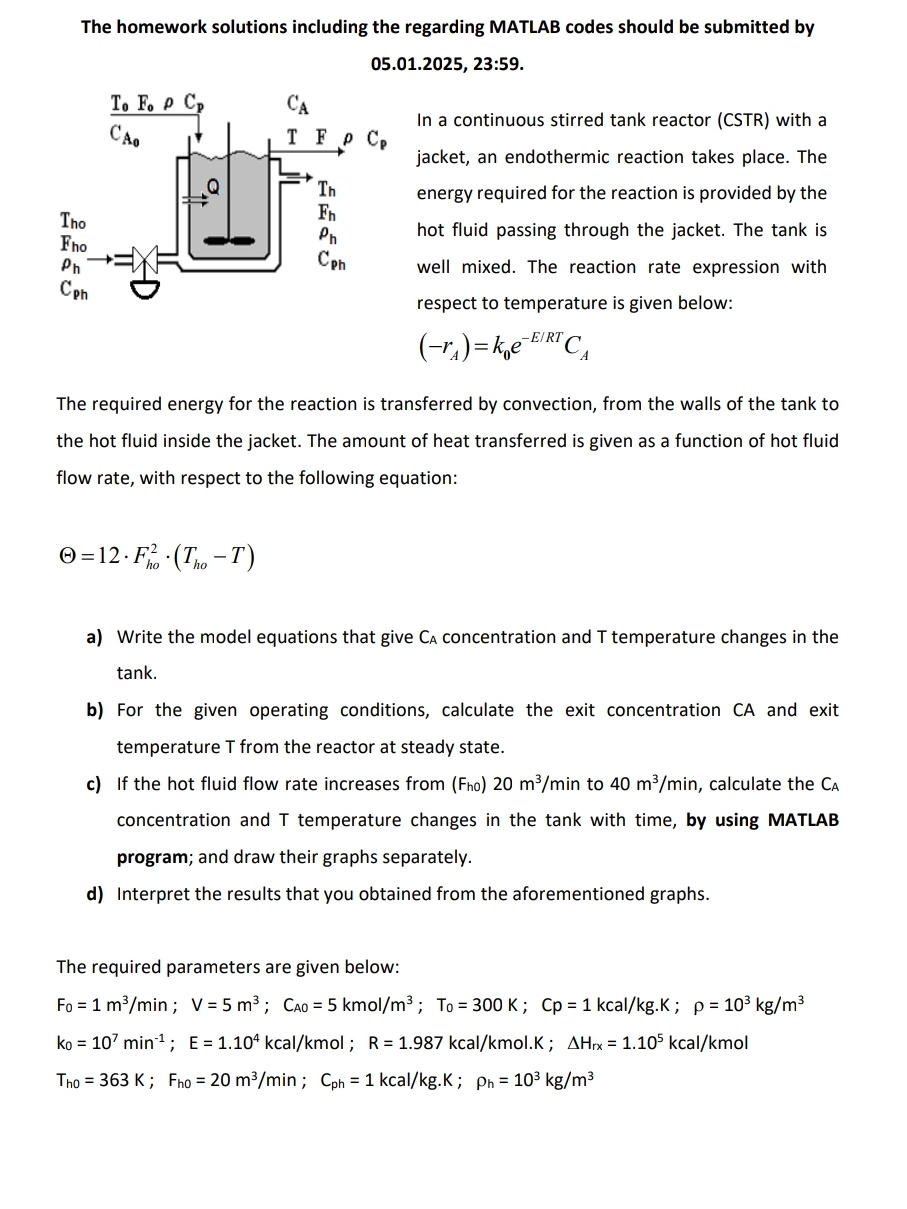
The homework solutions including the regarding MATLAB codes should be submitted by 05.01.2025, 23:59. In a continuous stirred tank reactor (CSTR) with a jacket, an endothermic reaction takes place. The energy required for the reaction is provided by the hot fluid passing through the jacket. The tank is well mixed. The reaction rate expression with respect to temperature is given below:
(-r_(A))=k_(0)e^(-(E)/(R)T)C_(A)The required energy for the reaction is transferred by convection, from the walls of the tank to the hot fluid inside the jacket. The amount of heat transferred is given as a function of hot fluid flow rate, with respect to the following equation:
\Theta =12*F_(ho)^(2)*(T_(ho)-T)a) Write the model equations that give
C_(A)concentration and
Ttemperature changes in the tank. b) For the given operating conditions, calculate the exit concentration CA and exit temperature T from the reactor at steady state. c) If the hot fluid flow rate increases from (
Fho_(h)to
40(m^(3))/(m)in, calculate the
C_(A)concentration and T temperature changes in the tank with time, by using MATLAB program; and draw their graphs separately. d) Interpret the results that you obtained from the aforementioned graphs. The required parameters are given below:
F_(0)=1(m^(3))/(m)in;V=5m^(3);C_(A0)=5kmo(l)/(m^(3));T_(0)=300K;Cp=1kca(l)/(k)g.K;\rho =10^(3)k(g)/(m^(3))
k_(0)=10^(7)min^(-1);E=1.10^(4)kca(l)/(k)mol;R=1.987kca(l)/(k)mol.K;\Delta H_(rx)=1.10^(5)kca(l)/(k)mol
T_(h0)=363K;F_(h0)=20(m^(3))/(m)in;C_(ph)=1kca(l)/(k)g.K;\rho _(h)=10^(3)k(g)/(m^(3))