Home /
Expert Answers /
Chemistry /
the-hydrogen-atom-bonded-to-the-central-carbon-nitric-acid-has-more-hydrogen-atoms-to-donate-which-pa132
(Solved): The hydrogen atom bonded to the central carbon Nitric acid has more hydrogen atoms to donate. Which ...
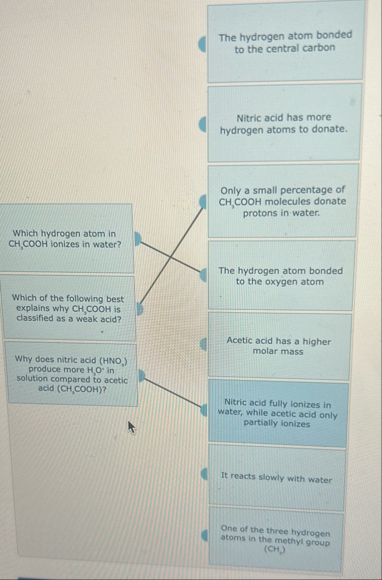
The hydrogen atom bonded to the central carbon Nitric acid has more hydrogen atoms to donate. Which hydrogen atom in
CH,(C)/(O)O Hionizes in water? Which of the following best explains why
CH,(C)/(O)O His dassified as a weak acid? Why does nitric acid (
HNO_(2)) produce more
H_(,)O^(')in solution compared to acetic acid
(CH,(C)/(O)O H)? Only a small percentage of
CH_(3)(C)/(O)O Hmolecules donate protons in water. The hydrogen atom bonded to the oxygen atom Acetic acid has a higher molar mass Nitric acid fully ionizes in water, while acetic acid only partially fonizes It reacts slowly with water One of the three hydrogen atoms in the methyl group (CH,)