Home /
Expert Answers /
Chemistry /
the-rate-constant-for-the-following-reaction-is-0-0620m1s1-aproductsrate-k-a-2-pa864
(Solved): The rate constant for the following reaction is 0.0620M1s1. Aproductsrate=k\[A]2 ...
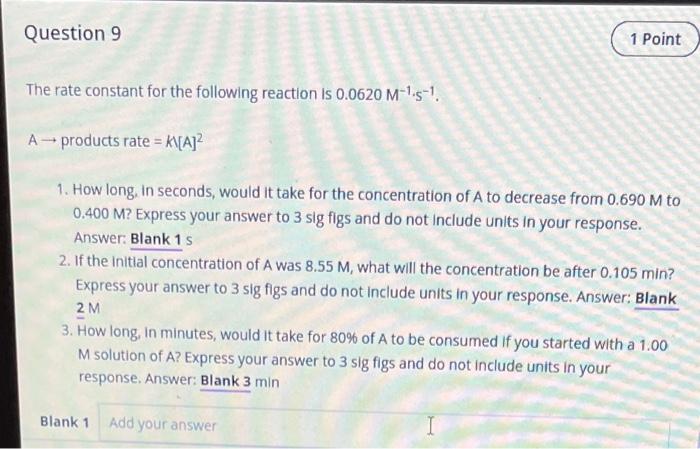
The rate constant for the following reaction is . 1. How long, in seconds, would it take for the concentration of A to decrease from to ? Express your answer to 3 sig figs and do not include units in your response. Answer: Blank 2. If the initial concentration of A was , what will the concentration be after ? Express your answer to 3 sig figs and do not include units in your response. Answer: Blank 3. How long, in minutes, would it take for of A to be consumed if you started with a M solution of A? Express your answer to 3 sig figs and do not include units in your response. Answer: Blank
Expert Answer
From the units of rate co