Home /
Expert Answers /
Chemistry /
the-reaction-between-potassium-chlorate-and-red-phosphorus-takes-place-when-you-strike-a-match-on-a-pa837
(Solved): The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a ...


The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react \( 40.1 \mathrm{~g} \) of potassium chlorate \( \left(\mathrm{KClO}_{3}\right) \) with excess red phosphorus, what mass of tetraphosphorus decaoxide \( \left(\mathrm{P}_{4} \mathrm{O}_{10}\right) \) could be produced? \[ \mathrm{KClO}_{3}(s)+\mathrm{P}_{4}(s) \rightarrow \mathrm{P}_{4} \mathrm{O}_{10}(s)+\mathrm{KCl}(s) \] (unbalanced) Mass \( = \)
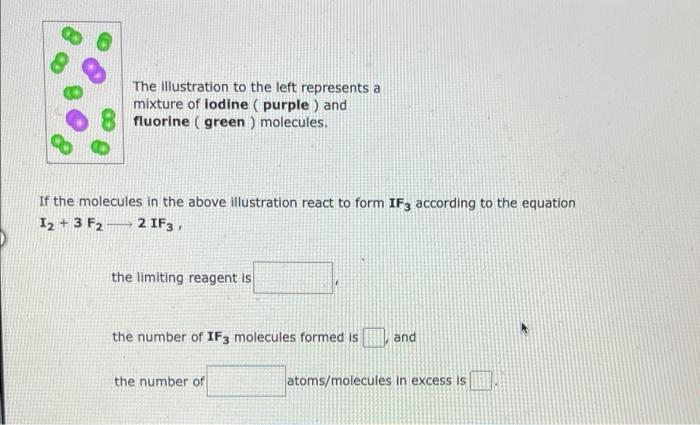
The Illustration to the left represents a mixture of lodine (purple) and fluorine (green ) molecules. If the molecules in the above illustration react to form \( \mathrm{IF}_{3} \) according to the equation \( \mathrm{I}_{2}+3 \mathrm{~F}_{2} \longrightarrow 2 \mathrm{IF}_{3} \) the limiting reagent is the number of \( \mathbf{I F}_{3} \) molecules formed is and the number of atoms/molecules in excess is
For the following reaction, \( 26.9 \) grams of carbon disulfide are allowed to react with 128 grams of chiorine gas. carbon disulfide \( (s)+ \) chlorine \( (g) \rightarrow \) carbon tetrachloride \( (l)+ \) sulfur dichloride \( (s) \) What is the maximum amount of carbon tetrachloride that can be formed? \[ \text { Mass = } \mathrm{g} \] What is the FORMULA for the limiting reactant? What amount of the excess reactant remains after the reaction is complete? \[ \text { Mass = } \]