Home /
Expert Answers /
Chemistry /
the-second-order-rate-constant-for-the-thermal-decomposition-of-no-according-to-the-following-reac-pa793
(Solved): The second order rate constant for the thermal decomposition of NO, according to the following reac ...
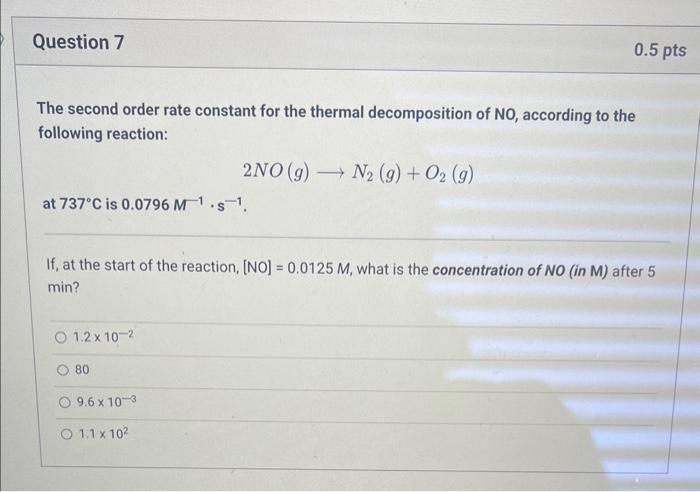
The second order rate constant for the thermal decomposition of NO, according to the following reaction: \[ 2 \mathrm{NO}(g) \longrightarrow \mathrm{N}_{2}(g)+\mathrm{O}_{2}(g) \] at \( 737^{\circ} \mathrm{C} \) is \( 0.0796 \mathrm{M}^{-1} \cdot \mathrm{s}^{-1} \). If, at the start of the reaction, [NO] \( =0.0125 \mathrm{M} \), what is the concentration of \( N O \) (in M) after 5 \( \min \) ? \( 1.2 \times 10^{-2} \) 80 \( 9.6 \times 10^{-3} \) \( 1.1 \times 10^{2} \)
Expert Answer
For secondary order reaction , we kn