Home /
Expert Answers /
Chemistry /
the-specific-heat-of-a-certain-type-of-metal-is-0-128-mathrm-j-left-mathrm-g-cdot-pa382
(Solved): The specific heat of a certain type of metal is \( 0.128 \mathrm{~J} /\left(\mathrm{g} \cdot{ }^{\ ...
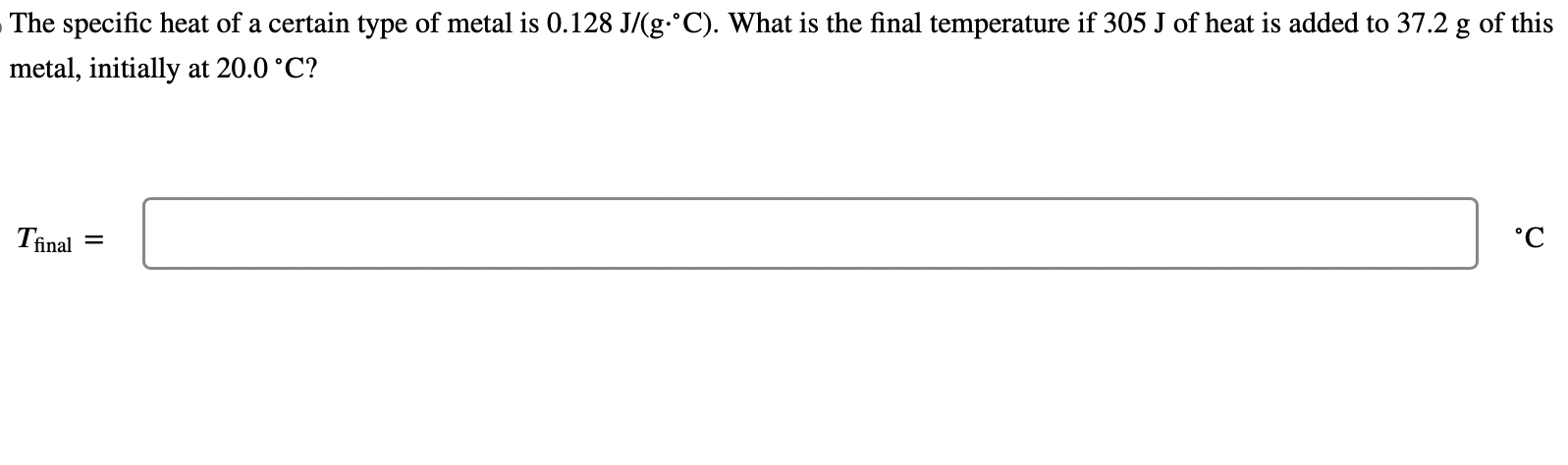
The specific heat of a certain type of metal is \( 0.128 \mathrm{~J} /\left(\mathrm{g} \cdot{ }^{\circ} \mathrm{C}\right) \). What is the final temperature if \( 305 \mathrm{~J} \) of heat is added to \( 37.2 \mathrm{~g} \) of this metal, initially at \( 20.0^{\circ} \mathrm{C} \) ? \( T_{\text {final }} \)
Expert Answer
Solution: Given data: -> specific heat, c = 0.128 J/ ( g.oC ) -> Heat, q =