(Solved): The value of K, for the reaction below is 9.53 at 457 K . NO_(2)(s)LongrightarrowN_(2)O_(4)(s) 2nd a ...
The value of
K, for the reaction below is 9.53 at 457 K .
NO_(2)(s)LongrightarrowN_(2)O_(4)(s)2nd attempt Wisee Periodic Table See Determine the reaction quotient for a mbare of the two pises in which
|NO_(2)|=0.0131Mand
|N,O_(4)|=0.00315M
◻Write the
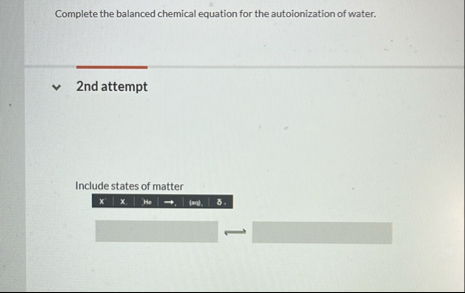
K_(3)expressions for the following two reactions. 2nd attempt Part 1 (1 point) Do not include states of matter, multiplication symbols, or extra spaces. Use brackets [] to indicate concentration. If the concentration of a substance should be " 1 ", then do not include it in the expression. Complete the Kexpression for the weak acid behavior represented by ( Complete the balanced chemical equation for the autoionization of water. 2nd attempt Include states of matter x x. He (ma) 8 . Quinine occurs naturally in the bark of the clinchona tree. For centurles it was the only treatment for malaria. Quinine contains two water.