Home /
Expert Answers /
Physics /
the-volume-of-an-ideal-gas-is-adiabatically-reduced-from-193-mathrm-l-mathrm-to-80-7-mathr-pa144
(Solved): The volume of an ideal gas is adiabatically reduced from \( 193 \mathrm{~L} \mathrm{to} 80.7 \mathr ...
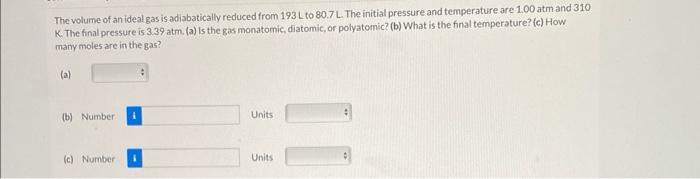
The volume of an ideal gas is adiabatically reduced from \( 193 \mathrm{~L} \mathrm{to} 80.7 \mathrm{~L} \). The initial pressure and temperature are \( 1.00 \) atm and 310 . K. The final pressure is \( 3.39 \mathrm{~atm} \). (a) is the gas monatomic, diatomic, or polyatomic? (b) What is the final temperature? (c) How many moles are in the gas? (a) (b) Number Units (c) Number Units
Expert Answer
(a) Let ?is the ratio of specific heats at constant pressure and constant volume. Let P,Vare the pressure and Volume of the ideal gas . For ideal gas