Home /
Expert Answers /
Chemistry /
this-is-a-precipiation-reaction-of-lead-ii-iodide-2-mathrm-ki-mathrm-aq-mathrm-pb-left-pa414
(Solved): This is a precipiation reaction of lead(II) iodide: \( 2 \mathrm{KI}(\mathrm{aq})+\mathrm{Pb}\left( ...

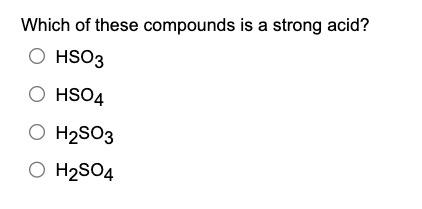
This is a precipiation reaction of lead(II) iodide: \( 2 \mathrm{KI}(\mathrm{aq})+\mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{aq}) \rightarrow \mathrm{Pbl}_{2}(\mathrm{~s})+2 \mathrm{KNO}_{3}(\mathrm{aq}) \) How many grams of lead(II) iodide will be created if \( 542 \mathrm{~mL} \) of \( 2.5 \mathrm{M} \) potassium iodide react completely? The molar mass of potassium iodide is \( 166.00 \mathrm{~g} / \mathrm{mol} \) and the molar mass of lead(II) iodide is \( 461.01 \mathrm{~g} / \mathrm{mol} \). Complete your calculation on paper using dimensional analysis. Circle the final answer with the correct number of significant figures, unit, and substance. Type your answer here with the correct number of significant figures, unit, and substance.
Which of these compounds is a strong acid? \( \mathrm{HSO}_{3} \) \( \mathrm{HSO}_{4} \) \( \mathrm{H}_{2} \mathrm{SO}_{3} \) \( \mathrm{H}_{2} \mathrm{SO}_{4} \)